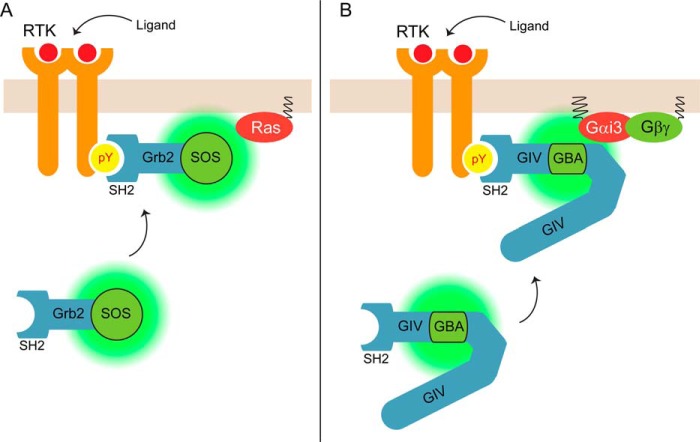
FIGURE 8.
Model depicting the parallelism between the mechanisms of activation of Gαi and Ras by their respective cytoplasmic GEFs, GIV, and SOS, upon RTK stimulation. A, under resting conditions, SOS is primarily located in the cytosol along with Grb2, whereas its substrate G protein Ras is constitutively anchored to the plasma membrane, thereby precluding SOS action. Upon RTK stimulation, Grb2-SOS complexes translocate to the plasma membrane via binding of Grb2 SH2 domains to tyrosine phosphorylated EGFR. This change of localization brings SOS in physical proximity to Ras, thereby promoting G protein activation. B, under resting conditions, GIV is primarily located in the cytosol, whereas its substrate G protein Gαi is constitutively anchored to the plasma membrane, thereby precluding GIV action. Upon RTK stimulation, GIV translocates to the plasma membrane via binding of its SH2-like domain to tyrosine phosphorylated EGFR. This change of localization brings GIV in physical proximity to Gαi, thereby promoting G protein activation.