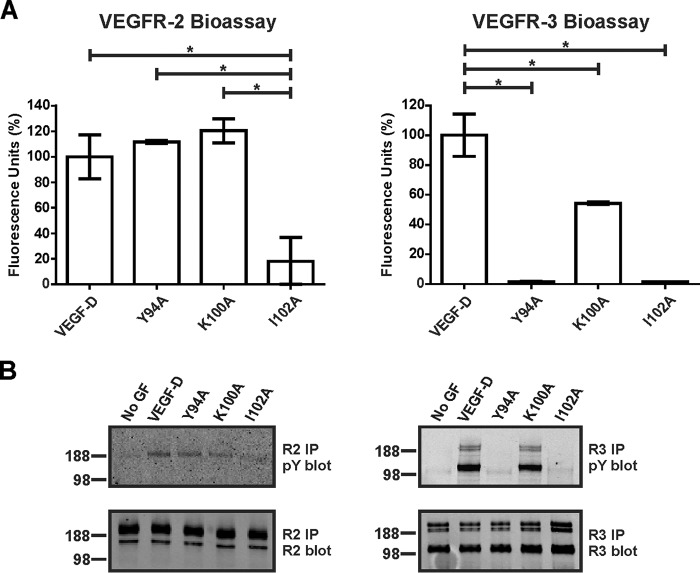
FIGURE 3.
Receptor binding and activation by untagged VEGF-D variants. A, bioassays for binding and cross-linking of extracellular domains of VEGFR-2 (left) and VEGFR-3 (right) with altered versions of VEGF-DΔNΔC, Y94A, K100A, and I102A lacking FLAG tag. The same amount of each VEGF-DΔNΔC variant was used. Results are expressed as a percentage of fluorescence units generated relative to untagged VEGF-DΔNΔC (y axis). VEGF-D, untagged form of VEGF-DΔNΔC. Assays were conducted three times. Columns, mean; error bars, S.D. *, statistically significant differences as assessed by one-way analysis of variance with Tukey's post hoc test. B, adult LECs were stimulated with matched quantities of untagged variants or left unstimulated (No GF). Lysates were immunoprecipitated (IP) with antibody against VEGFR-2 (left) or VEGFR-3 (right) and analyzed by reducing SDS-PAGE and Western blotting with antibody against phosphotyrosine (pY) to assess receptor activation (top blots) or with antibody against VEGFR-2 (bottom left blot) or VEGFR-3 (bottom right blot) to confirm the presence of each receptor. Sizes of molecular mass markers (in kDa) are shown to the left of the panels.