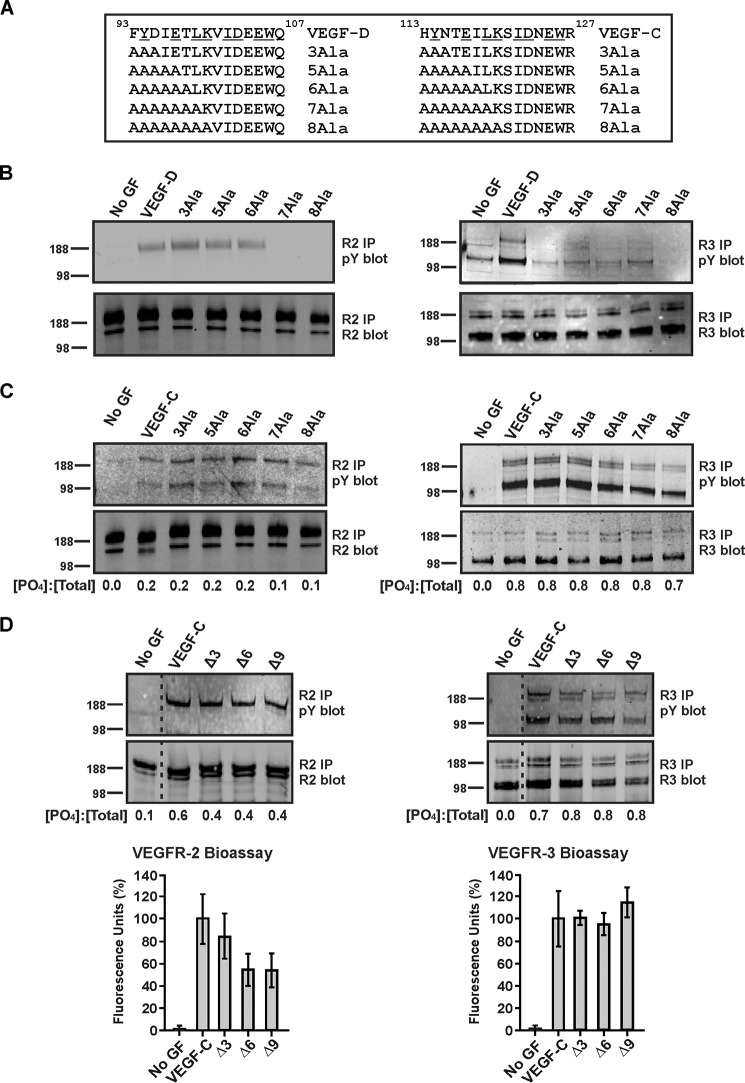
FIGURE 4.
Effects of mutating residues in N-terminal α-helices of VEGF-DΔNΔC or VEGF-CΔNΔC. A, sequences within the N-terminal α-helices of human VEGF-DΔNΔC (VEGF-D) and VEGF-CΔNΔC (VEGF-C) (top, with identical residues underlined) with variants in which multiple residues were altered to alanine shown below. B and C, blots show analyses of receptor phosphorylation by variants of VEGF-DΔNΔC and VEGF-CΔNΔC, respectively. D, blots show analyses of receptor phosphorylation induced by VEGF-CΔNΔC and mutants of VEGF-CΔNΔC lacking residues 113–115 (designated Δ3), 113–118 (Δ6), and 113–121 (Δ9). Graphs below blots show the results of bioassays of binding and cross-linking of VEGFR-2 and VEGFR-3 extracellular domains by VEGF-C variants (data are mean percentage of fluorescence relative to VEGF-CΔNΔC ± S.D.). For blots in B–D, adult LECs were stimulated with VEGF-DΔNΔC, VEGF-CΔNΔC or their variants or left unstimulated (No GF). Lysates were immunoprecipitated with antibody against VEGFR-2 (left-hand blots) or VEGFR-3 (right-hand blots) and analyzed by reducing SDS-PAGE and Western blotting with antibody against phosphotyrosine to assess receptor activation (top blots) or with antibody against VEGFR-2 (bottom left blots) or VEGFR-3 (bottom right blots) to confirm the presence of each receptor. Sizes of molecular mass markers (in kDa) are shown to the left of the blots. The amounts of VEGF-D or VEGF-C variants were matched in each experiment. Dotted lines indicate where irrelevant tracks have been excised from the images. In C and D, numbers under the lanes of blots represent the ratios of the intensities of phosphorylated receptor signals to intensities of total receptor signals ([PO4]/[total]) for each ligand treatment as determined by calculating the mean ratios from two independent experiments. The ratios for VEGFR-2 were derived by combining the intensities of the signals for bands in the size range of 188–230 kDa (note that the lower band of ∼125 kDa in the top left blot of C was not used because it probably represents co-immunoprecipitated VEGFR-3 arising from receptor heterodimers, as reported previously (68)), whereas those for VEGFR-3 are based on combining the intensities of the ∼125-, ∼175-, and ∼195-kDa forms of this receptor. pY, phosphotyrosine; IP, immunoprecipitation.