FIGURE 3.
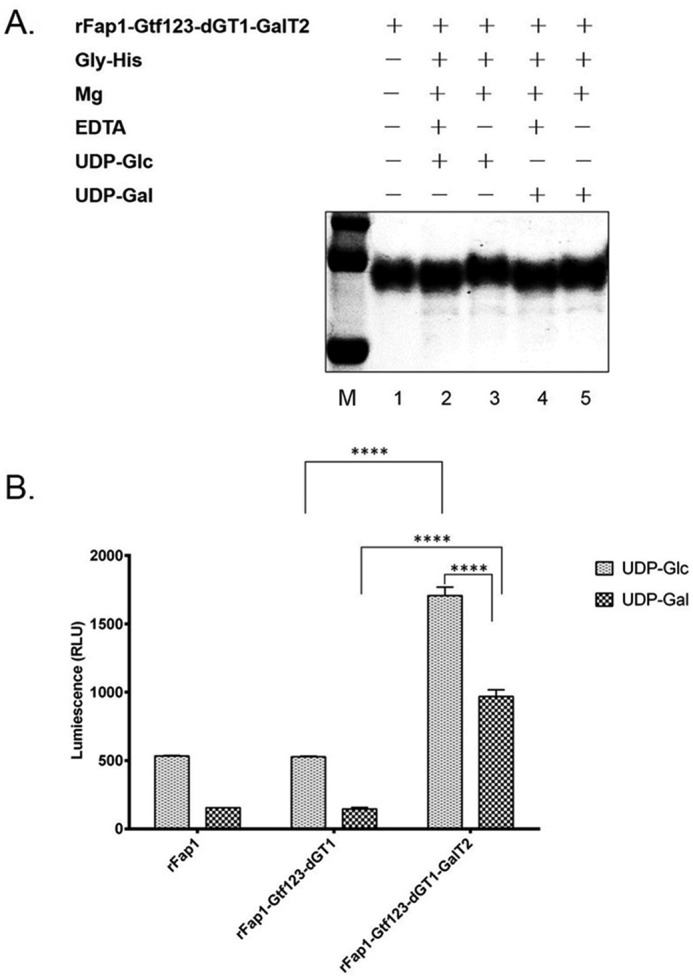
In vitro glycosyltransferase activity of Gly. A, in vitro glycosylation gel shift assay. Purified enzyme His-Gly and the substrate Gtf123-dGT1-GalT2 modified rFap1 were added into 50 μl of reaction buffer (20 mm Tris, pH 8.0, 100 mm NaCl, and 10 mm Mg2+). 10 mm EDTA was used to chelate metal ions. 10 mm UDP-Glc or UDP-Gal was used as a sugar donor. The reaction mixture was incubated in test tubes in a 37 °C water bath for 15 h. Completed reactions were then processed for SDS-PAGE and Coomassie blue staining. B, UDP-Glo glycosyltransferase assay. 5 μl of glycosyltransferase reaction mixtures that contain recombinant Gly enzyme, rFap1, or rFap1-Gtf123-dGT1, or rFap1-Gtf123-dGT1-GalT2 as a substrate and UDP-Glc or UDP-Galactose as a sugar donor were incubated in a solid white 384-well plate. After incubating with UDP detection reagent, luminescence was recorded using a BioTek microplate reader. The values represent the means of three replicates. RLU, relative light units. The bar graphs represent the means ± S.D. values. ****, p < 0.0001; two-tailed unpaired Student's t test.
