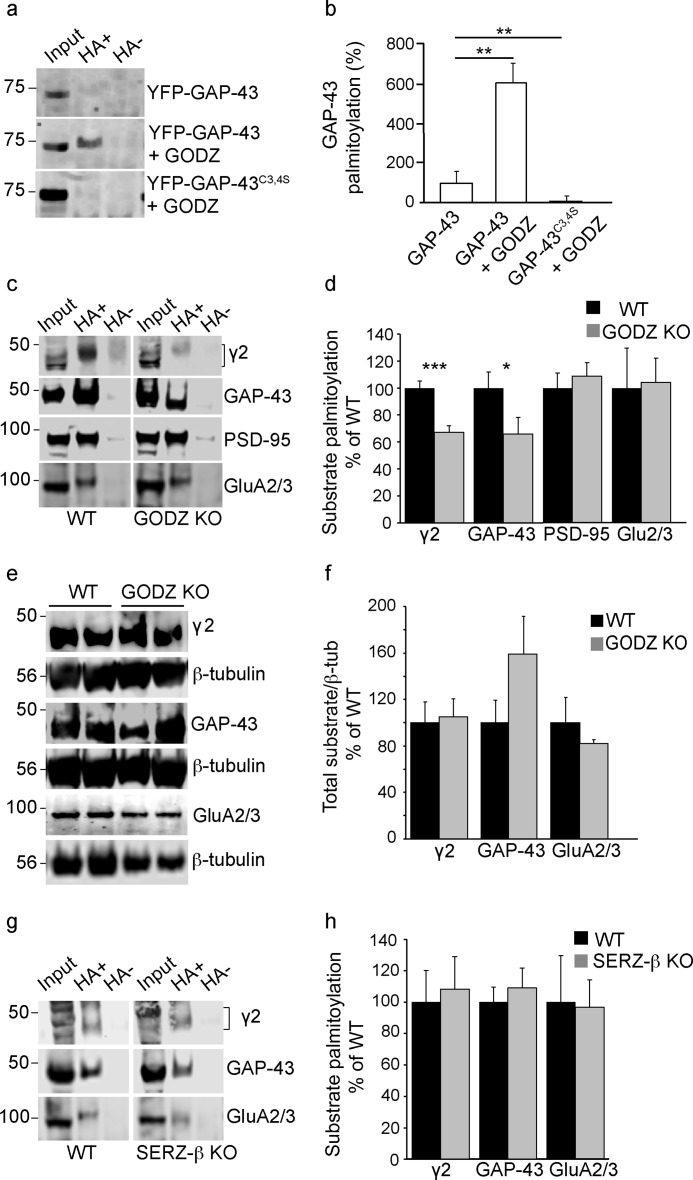
FIGURE 5.
GODZ KO mice exhibit reduced palmitoylation of GAP-43 and γ2-GABAARs. a, representative ABE assays of HEK293T cells transfected with either GFP-GAP-43 alone, GFP-GAP-43 with HA-GODZ, or GFP-GAP-43C3S,C4S with HA-GODZ. Input lanes represent aliquots of assays removed before pulldown of biotinylated samples and used for normalization. Hydroxylamine-containing (HA+) lanes represent palmitoylated substrate, and hydroxylamine-lacking (HA−) lanes represent negative controls (see “Experimental Procedures”). b, summary quantification of data in a showing GODZ-induced palmitoylation in a GAP-43 Cys-3/4-dependent manner. c and d, representative ABE assays of brain extracts from GODZ KO and WT mice analyzed on the same gel (c) and summary quantification of substrate palmitoylation normalized to input and WT (d). Note the marked reductions in steady state palmitoylation of the γ2 subunit and GAP-43 and unaltered palmitoylation of PSD-95 and GluA2/3 subunits in brain of GODZ KO versus WT mice. e and f, representative Western blots comparing total brain extracts of two GODZ KO and two WT mice probed for the γ2 subunit, GAP-43, or GluA2/3 subunits (e). The three proteins were analyzed in separate aliquots of the same extracts. Parallel blots of the same extracts were probed for β-tubulin (β-tub) and used to normalize γ2, GAP-43, and GluA2/3 protein amounts for summary quantification (f). Note that the expression of γ2, GAP-43, and GluA2/3 was unchanged except for a weak trend for increased expression of GAP-43 in KO versus WT brain. g and h, ABE assays of WT and SERZ-β KO brain extracts analyzed on the same gel and probed for γ2, GAP-43, or GluA2/3 (g). Summary statistics (h) indicate that palmitoylation of all three proteins was unchanged in SERZ-β KO versus WT brain. Bar graphs with error bars indicate means ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001, t tests.