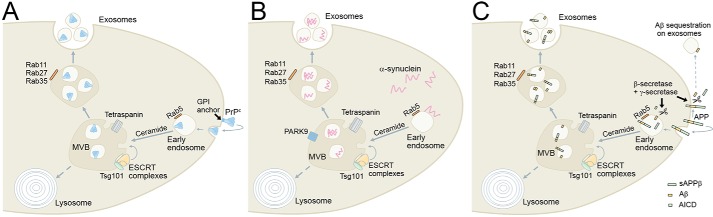
FIGURE 1.
Schematic depicting the pathways involved in the biogenesis of exosomes containing proteins associated with neurodegenerative diseases. A, the prion protein (PrPc) is found on the cell surface anchored by a glycosylphosphatidylinositol (GPI) linker to lipid rafts. PrPc can be internalized via early endosomes, and through a ceramide-dependent process (99), it can enter MVBs. MVB fusion with the cell surface results in the release of PrPc in exosomes. B, α-synuclein is a cytoplasmic protein that can be found on early endosomes. Through a process involving ceramide, tetraspanins, or endosomal sorting complexes required for transport (ESCRT) components, α-synuclein is directed into MVBs. Both PARK9 (51, 52) and Rab11 (48) have been shown to promote the exosomal release of α-synuclein. C, APP is a cell surface transmembrane protein that can be processed by a number of proteases. APP is found on early endosomes, where it can be cleaved by secretases (71) to form secreted APPβ (sAPPβ), Aβ, and APP intracellular domain (AICD). The AICD fragment can traffic to the nucleus, whereas Aβ can be trafficked into MVBs through a process involving ceramide, tetraspanins, or ESCRT components. Fusion of the MVB with the plasma membrane results in the subsequent release of exosomes containing Aβ from the cell. Exosome-independent pathways for Aβ release can result in extracellular Aβ that can be sequestered by exosomes, which can then be degraded by microglia (73).