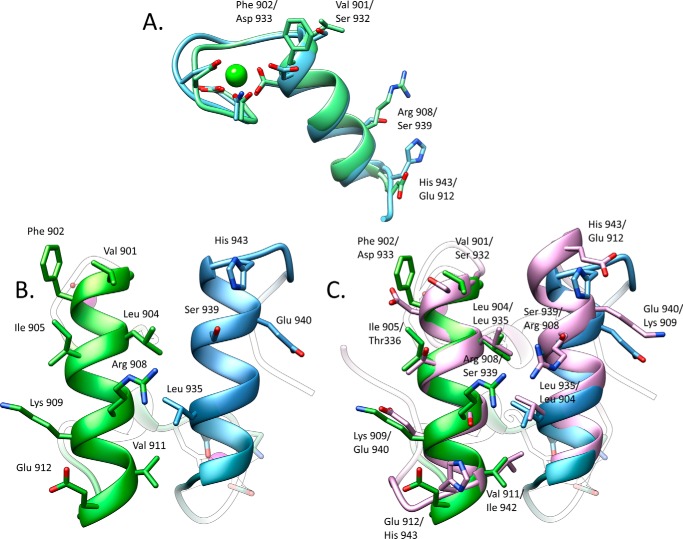
FIGURE 4.
Significant differences between the two cohesin-binding interfaces do not allow the dual binding mode of type I dockerin from R. flavefaciens. A, overlay of the two dockerin repeats observed in Doc3 showing that the structures are similar (r.m.s.d. of 0.82 Å) in the main chain atoms but have considerable differences in the side chains. B, two interacting helices of Doc3, helix 1 (bright green) and helix 3 (blue), with the most important cohesin recognition residues displayed as sticks. C, comparison of the two putative binding surfaces by overlaying Doc3 with a version of itself rotated by 180° (pink) shows a lack of conservation in the key contacting residues. Lack of internal symmetry in Doc3 and the involvement of the two helices in cohesin recognition suggest that Doc3 displays a single cohesin-binding platform.