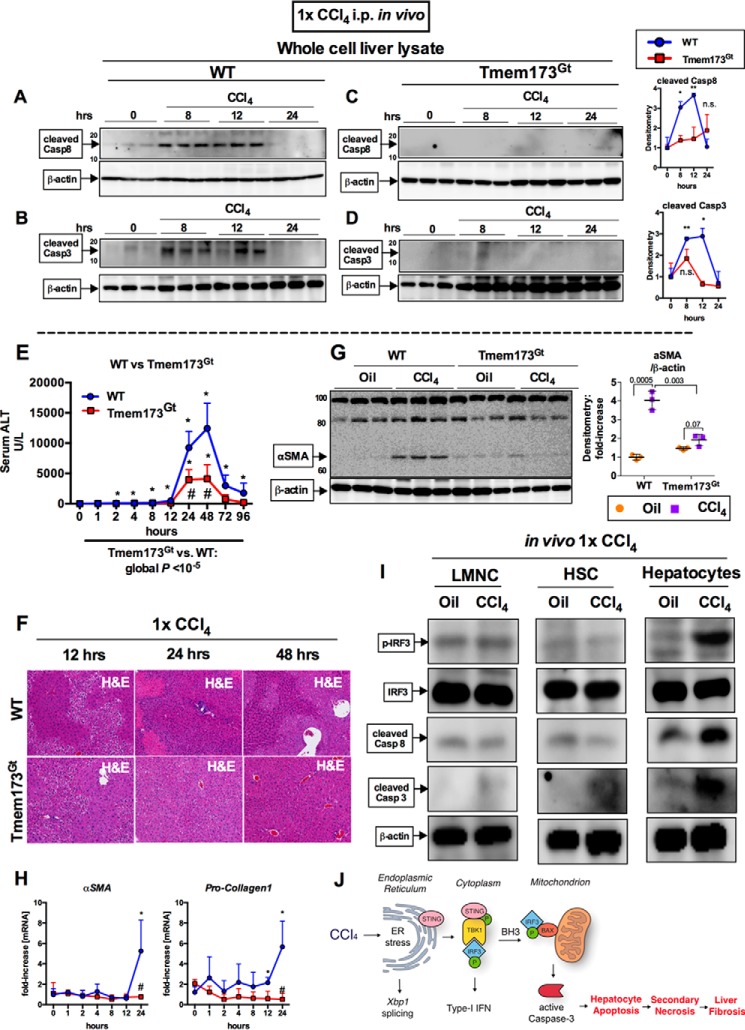
FIGURE 7.
Early pro-apoptotic activation of IRF3 by CCl4 is hepatocyte-specific and mediated by STING. WT or STING-deficient mice (Tmem173Gt) received a single injection of CCl4 or oil (baseline) and sacrificed at indicated time points. A–D, whole cell liver lysates were probed for cleaved caspase-8 (A and B) and cleaved caspase-3 (C and D) by immunoblot. E and F, liver injury was assessed by serum ALT (E) and H&E staining at the indicated time points (F). G and H, deposition of α-SMA 24 h after injection was evaluated by immunoblots from whole cell liver lysate (G), whereas mRNA expression in liver for Acta2 and Col1a2 (H) was assessed by PCR. n = 5–8 mice per time point, per genotype (A–H). I, WT mice received a single injection of CCl4 or oil (baseline) and sacrificed 9 h later. LMNCs, HSCs, or hepatocytes were isolated from livers and probed for phosphorylated IRF3, total IRF3, cleaved caspase-8, and cleaved caspase-3 by immunoblot. n = 3 per condition (LMNCs and HSCs were pooled from 3 mice); n = 1 (hepatocytes isolated from single mouse per condition); the experiment was repeated three times. J, schematic of pro-apoptotic STING and IRF3 activation from CCl4 administration in mice. In CCl4-treated hepatocytes, ER stress results in phosphorylation of TBK1 via STING, followed by phosphorylation of IRF3. IRF3 associates with BAX in the mitochondria through its BH3-only domain, leading to pro-apoptotic caspase-3 activation and hepatocyte apoptosis. After chronic CCl4 administration, hepatocyte apoptosis is associated with secondary necrosis, which results in liver fibrosis.