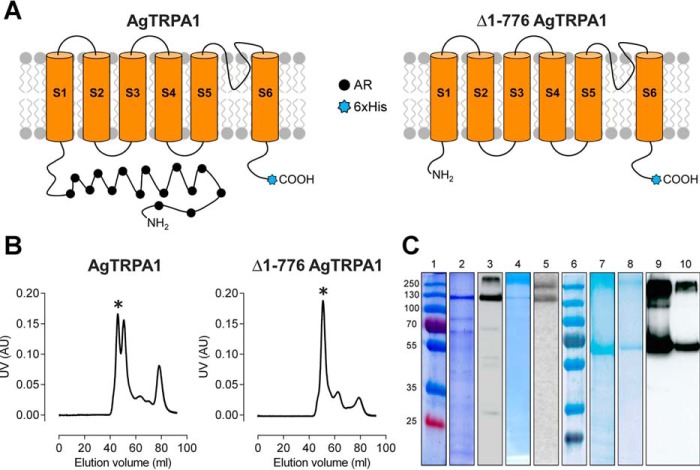
FIGURE 1.
Purification of full-length AgTRPA1 and N-terminally truncated (Δ1–776 AgTRPA1) constructs. A, schematic representation of AgTRPA1 and Δ1–776 AgTRPA1 showing the N-terminal truncation, the six transmembrane helices (S1–S6) with the pore region connecting the last two helices (S5 and S6), and the N and C termini facing the cytoplasm. The N-terminal containing ankyrin repeats (AR; filled black circles) and C-terminal hexa-His tag (6xHis; blue stars) are also indicated. B, purification profile of AgTRPA1 and Δ1–776 AgTRPA1 using a Superdex 200 16/26 column with the tetrameric fraction indicated by an asterisk. C, SDS-PAGE and Western blotting of AgTRPA1 and Δ1–776 AgTRPA1. The panel is assembled from several gels and blots; thus, two molecular mass markers are shown. Lanes 1 and 6, molecular mass markers (in kDa); lanes 2 and 3, AgTRPA1 after Ni-NTA affinity chromatography; lanes 4 and 5, tetrameric fraction after size exclusion chromatography. Lanes 7 and 9, Δ1–776 AgTRPA1 after Ni-NTA affinity chromatography; lanes 8 and 10, tetrameric fraction after size exclusion chromatography. Two major bands are seen in Western blots of the purified proteins (lanes 5 and 10) corresponding to monomeric and multimeric forms of the proteins.