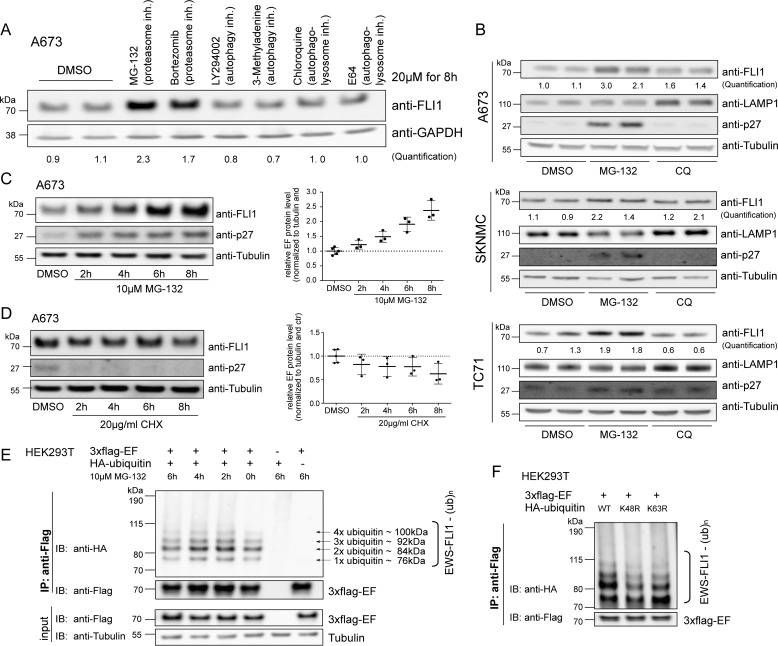
FIGURE 1.
EWS-FLI1 protein turnover is proteasome-dependent. A, Western blotting analysis of EWS-FLI1 protein levels. A673 cells were treated with a 20 μm concentration of the indicated compounds for 8 h, and EWS-FLI1 protein levels were detected with anti-FLI1 antibody. Quantification represents the ratio of FLI1 over GAPDH compared with DMSO control. B, EWS-FLI1 turnover in various Ewing sarcoma cell lines. A673, SKNMC, and TC71 cells were treated with 20 μm MG-132 or chloroquine (CQ) for 10 (A673), 8 (SKNMC), and 9 h (TC71) and immunoblotted with anti-FLI1 antibody. Quantification represents the ratio of FLI1 over tubulin compared with DMSO control. C, EWS-FLI1 stabilizes in a time-dependent manner. Shown is a Western blot of A673 cells treated with 10 μm MG-132 for the indicated time points. Three independent experiments were quantified and are represented in the scatter plot with n = 3 (2–8 h) or n = 5 (DMSO); error bars represent S.D. D, half-life of endogenous EWS-FLI1 protein. Shown is a Western blot of A673 cells treated with 20 μg/ml CHX for the indicated hours. Three independent experiments were quantified with n = 3 (2–8 h) or n = 5 (DMSO); error bars represent S.D. E, EWS-FLI1 is ubiquitinated. 3xFLAG-EWS-FLI1 and HA-ubiquitin were co-expressed for 48 h in HEK293T cells and incubated with 10 μm MG-132 for the indicated hours. After immunoprecipitation of EWS-FLI1 with anti-FLAG, ubiquitination was visualized by anti-HA antibody. F, EWS-FLI1 ubiquitination consists of Lys-48-linked ubiquitin chains. 3xFLAG-EWS-FLI1 and wild type or mutant HA-ubiquitins were co-expressed for 48 h in HEK293T cells and immunoprecipitated, and ubiquitination was visualized by anti-HA antibody. inh., inhibitor; IP, immunoprecipitation; IB, immunoblotting; ub, ubiquitin; EF, EWS-FLI1.