FIGURE 3.
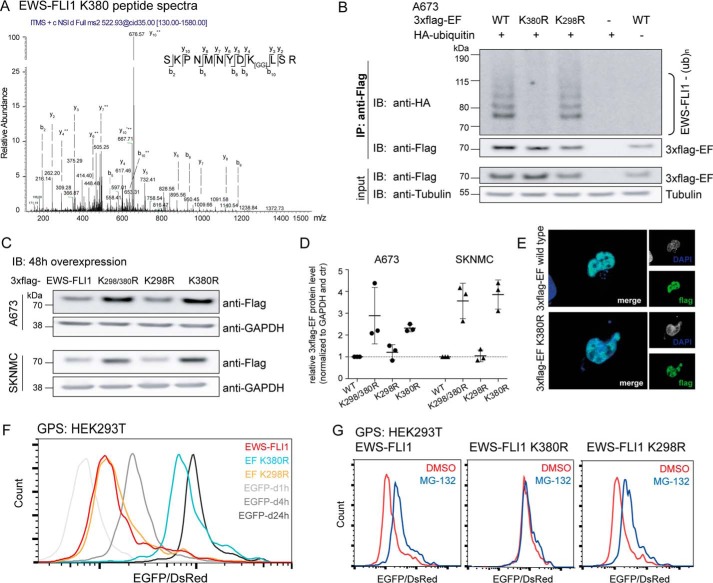
Mass spectrometry identifies Lys-380 residue as the main ubiquitination site. A, two lysine residues were identified to be ubiquitinated by mass spectrometry. Peptide spectra with a glycine-glycine shift for residue Lys-380 (peptide spectra for the Lys-298 site in supplemental Fig. S1A). B, mutation of lysine residue Lys-380 prevents EWS-FLI1 ubiquitination. 3xFLAG-EWS-FLI1 and K298R and K380R mutants were co-expressed with HA-ubiquitin for 48 h in A673 cells, and purified ubiquitinated EWS-FLI1 was analyzed using anti-HA antibody by Western blotting. C, mutated Lys-380 residue stabilizes EWS-FLI1 protein. 3xFLAG-EWS-FLI1 and single and double mutants were transiently overexpressed for 48 h in A673 and SKNMC cells and analyzed with an anti-FLAG antibody by Western blotting. D, scatter plot for quantification for n = 3 independent experiments; error bars represent S.D. E, EWS-FLI1 mutant shows nuclear localization. 3xFLAG-EWS-FLI1 and K380R single mutant were transiently expressed for 48 h in A673 cells. Cells were fixed, stained with anti-FLAG antibody, and used for immunofluorescence (40× magnification). F, mutation of Lys-380 stabilizes EWS-FLI1 posttranslationally. HEK293T cells were transduced for 72 h with reporter constructs of EWS-FLI1, mutants K298R and K380R, and standards. EGFP/DsRed ratios were analyzed by FACS (G) after additional incubation with DMSO or 20 μm MG-132 for 8 h. IP, immunoprecipitation; IB, immunoblotting; EF, EWS-FLI1; ub, ubiquitin; d1h, d4h, and d24h, degron (d) motifs with half-lives of 1, 4, and 24 h, respectively.