FIGURE 3.
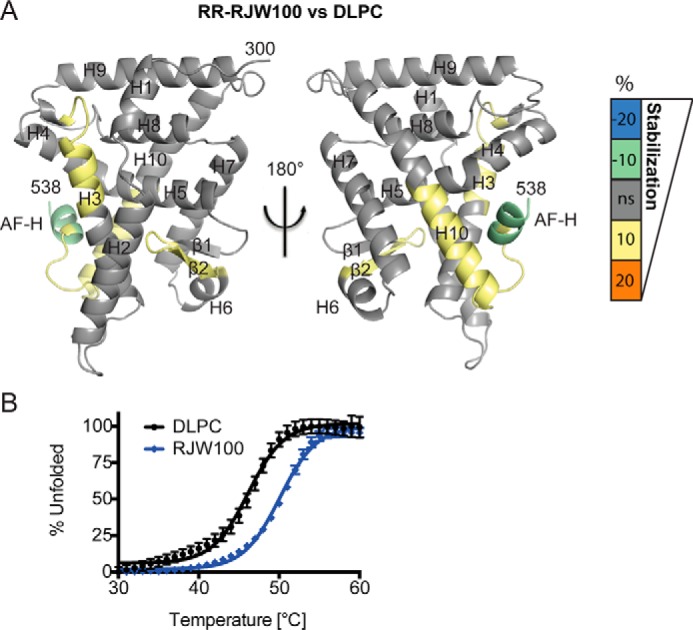
Differential effects on LRH-1 dynamics when RR-RJW100 is bound versus DLPC. A, HDX was used to probe differential effects on protein dynamics by the two ligands. The scale refers to the difference in percent deuterium incorporation for RR-RJW100-bound LRH-1 minus DLPC-bound LRH-1. The scale reflects the difference in average percent deuterium incorporation for RR-RJW100-bound versus DLPC-bound LRH-1 LBD. For example, negative numbers reflect slower deuterium incorporation (less motion) for RR-RJW100 versus DLPC. Results are mapped onto PDB 4DOS (20). B, DSF curves showing that RJW100 increases overall LRH-1 thermostability compared with DLPC. Each point represents the mean ± S.E. of values for three independent experiments, each conducted in triplicate.
