FIGURE 8.
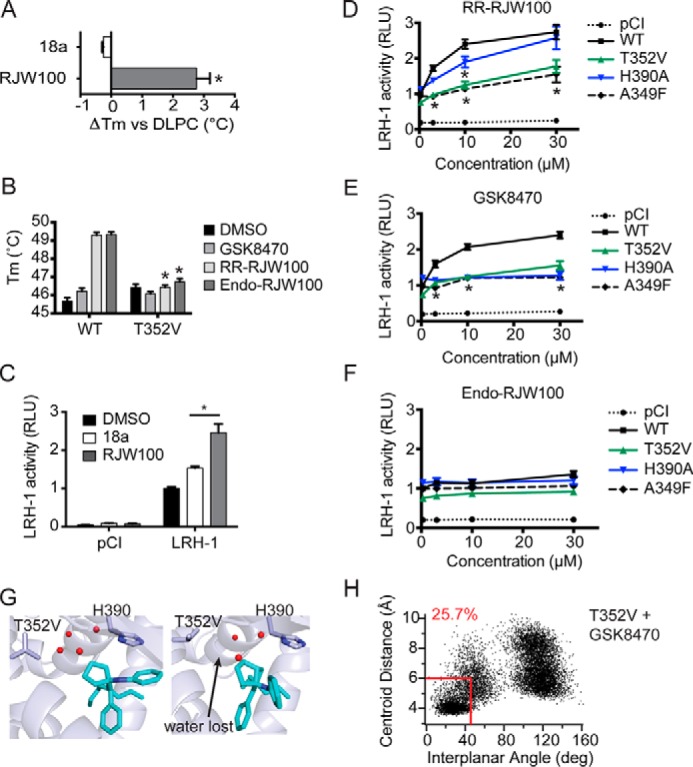
Importance of protein-ligand interactions on ligand binding and activity. A, analog 18a, lacking the hydroxyl group, does not stabilize wild-type LRH-1 in DSF assays. B, introduction of the T352V mutation to LRH-1 ablates the stabilizing effects of RR-RJW00 and endo-RJW100. Purified LRH-1 LBD, initially bound to DLPC for homogeneity, was incubated with either DMSO (control) or synthetic agonist dissolved in DMSO. C, compound 18a is a significantly weaker agonist in luciferase reporter assays. D–F, luciferase reporter assays measuring LRH-1 activity, using the SHP-luc reporter. Values have been normalized to constitutive Renilla luciferase signal and are presented as fold change versus wild-type LRH-1 + DMSO. The A349F mutation introduces a bulky aromatic side chain, which blocks the binding pocket and prevents binding of synthetic ligands (27). This was used as a negative control. G, snapshots from MDS using T352V LRH-1 with GSK8470 bound. H, plot of distances between ring centroids (x axis) and angle between the ring planes (y axis) for the GSK8470 phenyl group and His-390 at each time increment of the 200 ns with T352V LRH-1 (as described for Fig. 7). A–F, each bar (or point, for D–F) represents the mean ± S.E. for three independent experiments, each conducted in triplicate. *, p < 0.05 (significance was determined by two-way analysis of variance followed by Tukey's multiple comparisons test).
