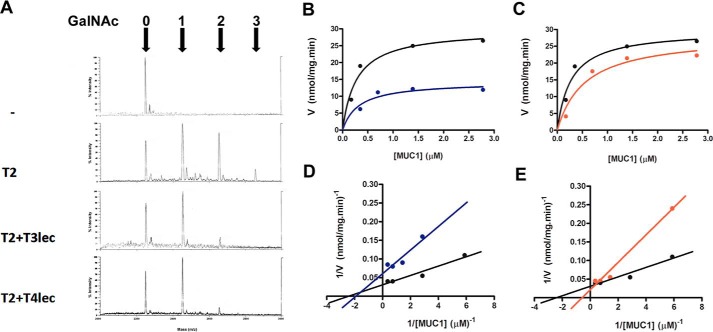
FIGURE 1.
Effects of lectin domains on enzyme activity of ppGalNAc-T2 (T2). A, MUC1 glycosylation product was analyzed by MALDI-TOF. Numbers of α-GalNAc incorporated were determined on acceptor peptide without ppGalNAc-T2 enzyme (control), with ppGalNAc-T2 (8 nm), and with ppGalNAc-T2 (8 nm) in the presence of 1.6 μm T3lec or T4lec domain. B–E, kinetics plots of MUC1 peptide glycosylation assay under initial velocity condition of ppGalNAc-T2 (1.6 nm) enzymatic reaction (black). Effects of 0.16 μm T3lec (B, blue) and T4lec (C, red) on ppGalNAc-T2 activity were assayed. Plots were fitted to the Michaelis-Menten equation using the GraphPad software program, yielding R2 values of 0.94 (black), 0.81 (blue), and 0.94 (red). Double reciprocal plots of enzyme activity without lectin domain (black) or in the presence of 0.16 μm T3lec (D, blue) or T4lec (E, red) indicated the type of inhibition of ppGalNAc-T2 activity. Plots were fitted to linear regression using the GraphPad software program, yielding R2 values of 0.94 (black), 0.88 (blue), and 0.98 (red). Results are representative of three independent experiments.