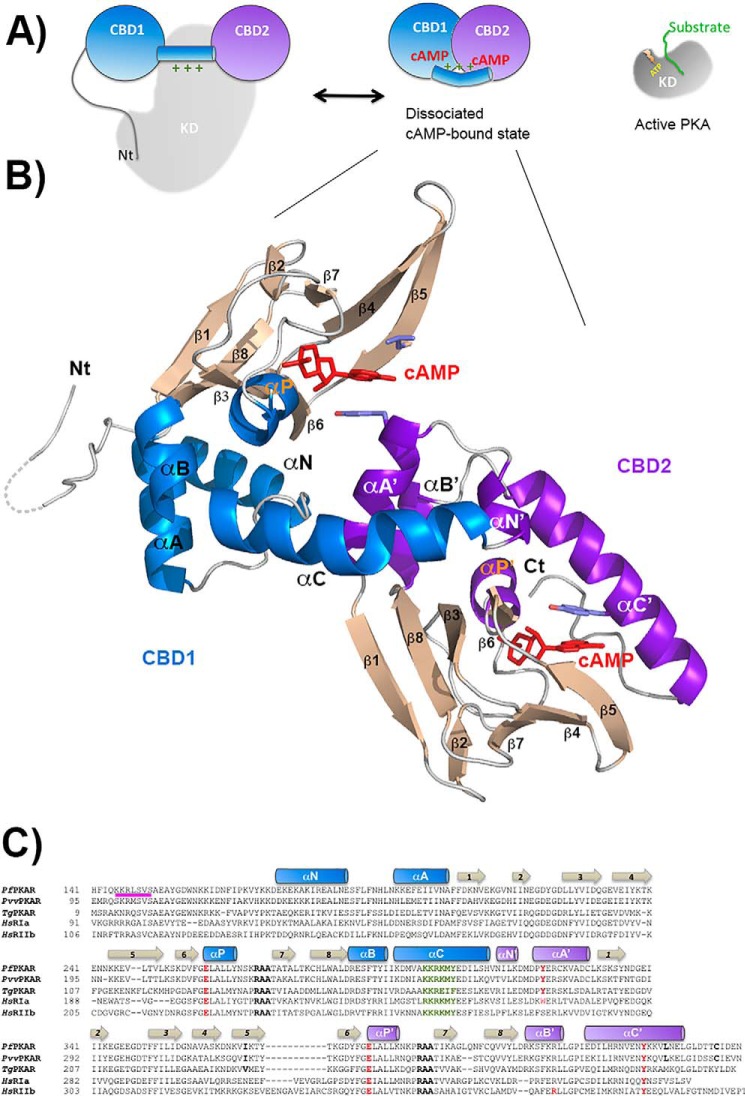
FIGURE 3.
Structure of PfPKA-R. A, schematic representation of the mammalian PKA holoenzyme and its activation mechanism. Catalytic domain depicted in gray and CBD1 and CBD2 domains in blue and purple. B, schematic representation of the nucleotide-bound PfPKA-R structure. β-Strands are colored tan, CBD1 α-helices blue, and CBD2 α-helices purple. All secondary structure elements are labeled within each CBD. The two bound cAMP molecules are shown in red. C, sequence alignment of the residues within the respective CBD domains for the PKA-R subunits from P. falciparum, Plasmodium vinckei vinckei, Toxoplasma gondii, and Homo sapiens RIα and RIIβ. The conserved PBC arginines are highlighted in black, the αP glutamates and the tyrosine with which they interact in red, and the inhibitory sequence in pink.