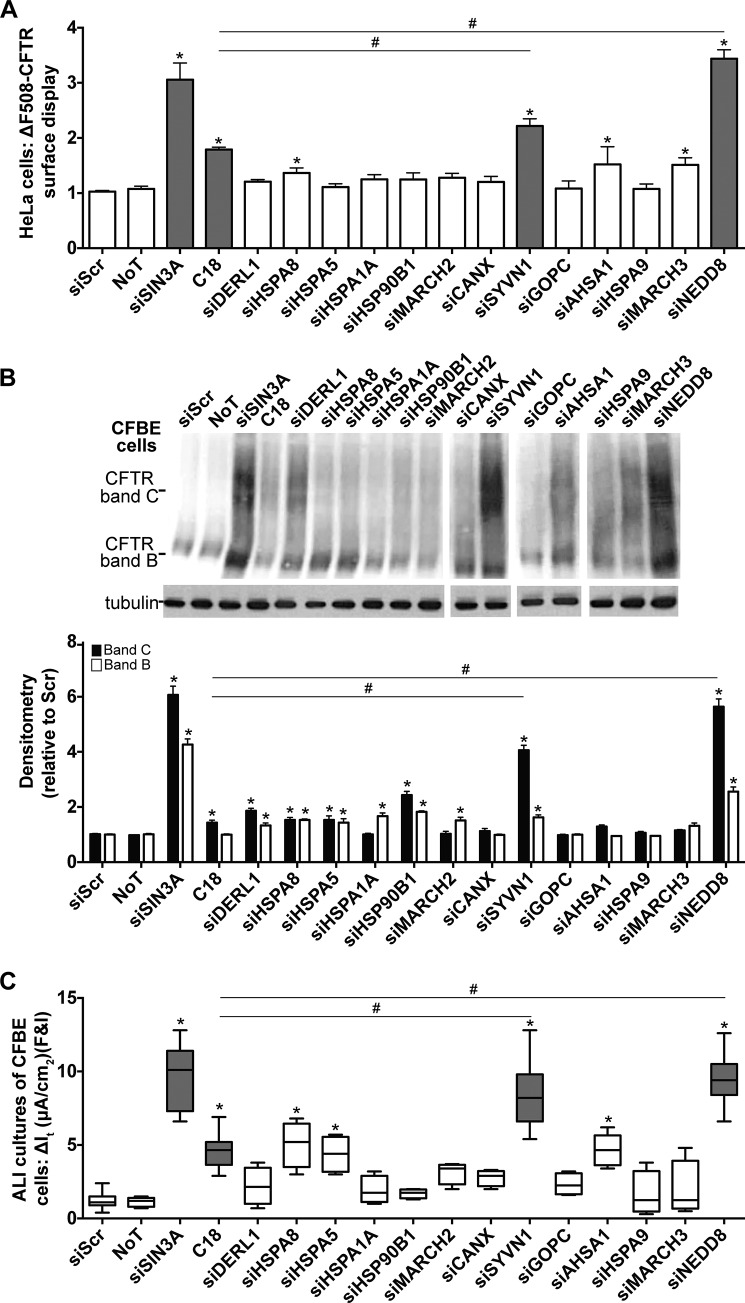
FIGURE 2.
ΔF508-CFTR biosynthesis is regulated by SYVN1 and NEDD8. A, HeLa-ΔF508-CFTR-HA cells were transfected with the DsiRNAs targeting each candidate gene. 24 h post-transfection, ΔF508-CFTR surface display was measured using an anti-HA antibody. As negative controls, cells were transfected with siScr or left untreated (no treatment (NoT)). As positive controls, SIN3A expression was depleted with a DsiRNA, or cells were treated with the corrector compound C18 for 24 h (6 μm). -Fold increase and significance are plotted relative to siScr transfection. C18 (6 μm) was administered for 24 h. n = 48. B, representative immunoblot depicting ΔF508-CFTR expression in CFBE cells. CFBE cells were transfected with the DsiRNAs targeting each candidate gene. As negative controls, cells were transfected with siScr, or left untreated (no treatment (NoT)). As positive controls, SIN3A expression was depleted with a DsiRNA, or cells were treated with the corrector compound C18 for 24 h (6 μm). Protein was harvested 72 h post-treatment. Densitometry represents -fold increase of ΔF508-CFTR bands C and B in CFBE cells relative to siScr. n = 4. C18 (6 μm) was administered for 24 h. C, change in It in response to forskolin and IBMX (F&I) treatment in polarized ALI cultures of CFBE cells. n = 8 (minimum) cultures per treatment. C18 (6 μm) was administered basolaterally 24 h prior to electrophysiology study. A and B represent mean with error bars indicating S.E. C, box and whisker plot (minimum-maximum) represents median. Statistical significance was determined by one-way ANOVA with Holm-Bonferroni correction: *, p < 0.01 (relative to siScr); #, p < 0.01.