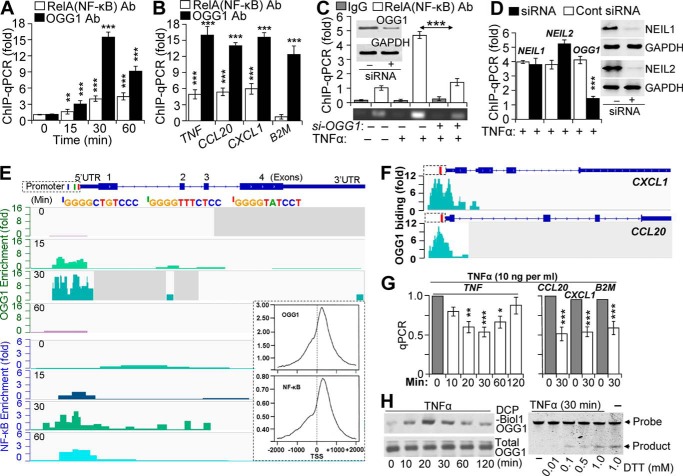
FIGURE 1.
Binding of NF-κB to promoters is OGG1-dependent. A, TNFα-induced enrichment of OGG1 and NF-κB on TNF promoter. Cells expressing FLAG-OGG1 were mock- or TNFα-exposed, and ChIP was performed at 0, 15, 30, and 60 min using anti-FLAG(OGG1) or anti-RelA(NF-κB) Ab. Uncross-linked DNA was subjected to PCR amplification as described under “Experimental Procedures” (n = 4). B, TNFα-induced enrichment of OGG1 and NF-κB on CCL20, CXCL1, and B2M promoters. Cells expressing FLAG-OGG1 were mock- or TNFα-exposed, and ChIP was performed at 30 min using anti-FLAG(OGG1) or anti-RelA(NF-κB) Ab. Uncross-linked DNA was subjected to PCR amplification of TNF, CCL20, CXCL1, and B2M promoters as described under “Experimental Procedures.” C, OGG1 expression-dependent enrichment of NF-κB on TNF promoter. OGG1 expression was down-regulated by siRNA (scrambled siRNA was used as a control, see under “Experimental Procedures”), and cells were TNFα-exposed. ChIP was performed at 30 min using anti-RelA(NF-κB) Ab (in controls, IgG). The levels of the TNF promoter in ChIP-ed DNA were determined by qPCR. The upper panel is a graphical depiction of changes in NF-κB binding to the TNF promoter in the presence and absence of OGG1 (n = 3). Lower panel, ethidium bromide-stained agarose gel from a representative experiment. Inset, cells were transfected with control and target-specific (OGG1) siRNA. At 48 h thereafter, cells were lysed to prepare protein extracts, and levels of OGG1 were determined by Western blotting. D, OGG1 depletion, but not that of NEIL1 or NEIL2, decreases NF-κB's association with promoters. NEIL1, NEIL2, or OGG1 expression was down-regulated by siRNA as described under “Experimental Procedures,” and cells were TNFα-exposed. ChIP was performed using Ab to RelA(NF-κB). The levels of TNF promoter in ChIP-ed DNA were determined by qPCR (30-min time point is shown). E, enrichment of OGG1 and NF-κB on the TNF promoter. FLAG-OGG1-expressing cells were TNFα-exposed and ChIP-ed at 0, 15, 30, and 60 min using Abs against RelA(NF-κB) or FLAG(OGG1). ChIP-ed DNA was subjected to sequencing, and sequence data were analyzed as described under “Experimental Procedures.” Images are directly taken from the Integrative Genomics Viewer. Enrichment levels (fold) of OGG1 and NF-κB for each time points are shown (left-hand side). Blue, green, and red bars are positions of NF-κB-binding motifs in promoter. Inset, distribution of OGG1 and NF-κB on TSS-adjacent sequences ±2000 bp at whole genome level. F, enrichment of OGG1 at the CXCL1 and CCL20 promoters. FLAG-OGG1-expressing cells were TNFα-exposed and ChIP-ed using Ab FLAG(OGG1), and ChIP-ed DNA was sequenced (see under “Experimental Procedures”). Images show enrichment peaks of OGG1 at 30 min after TNFα exposure of cells. Images are directly taken from the Integrative Genomics Viewer. Red bars within CXCL1 and CCL20 promoter regions are the locations of NF-κB-binding motifs. G, OGG1 digestion decreases qPCR product levels of TNF, CCL20, CXCL1, and B2M promoters. Cells were exposed to TNFα (20 ng/ml) for various lengths of time, and then the isolated DNAs were OGG1-digested and subjected to qPCR as under “Experimental Procedures” (n = 3). H, TNFα exposure of cells induces oxidative modifications of OGG1 and decreases its enzymatic activity. FLAG-OGG1-expressing cells were TNFα-exposed, and nuclear extracts were made in the presence or absence of DCP-Bio1 (a cysteine sulfenic acid-reacting agent). Levels of DCP-Bio1-tagged OGG1 were determined by streptavidin-coupled chemiluminescence. Total OGG1 levels were determined by immunoblotting using Ab to FLAG (OGG1). Lower panel: enzymatic activity of OGG1 is decreased in TNFα-exposed cells. NE was isolated at the 30-min post-exposure, and OGG1's excision activity was determined in the presence or absence of DTT in the reaction buffer, as described under “Experimental Procedures.” * = p < 0.05; ** = p < 0.01; *** = p < 0.001; Cells used are as follows: HEK293; CCL20, promoter of that chemokine (C-C motif) ligand 20 gene; CXCL1, promoter of chemokine (CXC motif) ligand 1 gene; B2M, promoter of β2-microglobulin gene; NEIL1, gene human Nei-like DNA glycosylase-1; NEIL2, gene human Nei-like DNA glycosylase 2; OGG1, gene of human 8-oxoguanine DNA glycosylase.