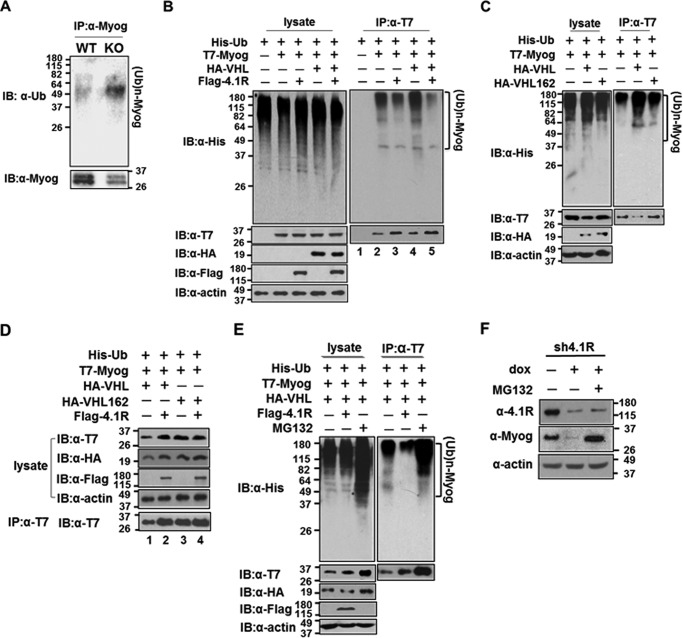
FIGURE 10.
4.1R inhibits VHL-mediated ubiquitination and myogenin degradation via the ubiquitin-proteasome pathway. A, 4.1R knockdown increases polyubiquitinated myogenin. WT-MyoD and KO-MyoD MEF cells were cultured in differentiation medium in the presence of doxycycline for 48 h and analyzed for myogenin levels and its status of ubiquitination. Top, ubiquitination of myogenin was analyzed by immunoprecipitation (IP) of cell lysates with an anti-myogenin Ab and immunoblotting (IB) with an anti-Ub antibody. (Ub)n-MyoG, ubiquitinated myogenin. Bottom, the amount of immunoprecipitated myogenin was detected using an anti-myogenin Ab. B, decrease in polyubiquitinated myogenin by 4.1R overexpression. HEK-293 cells were transfected with combinations of His-Ub, T7-Myog, HA-VHL, and FLAG-4.1R as indicated. Left panel, lysates fractionated on an SDS-polyacrylamide gel were blotted with an anti-His antibody. The presence of the exogenously expressed proteins in cell lysates was confirmed by immunoblotting with an anti-T7 Ab for myogenin, anti-HA for VHL, and anti-FLAG for 4.1R. Actin served as a loading control. Right, extracts were immunoprecipitated with an anti-T7 antibody and immunoblotted with an anti-His Ab. The presence of T7-myogenin was confirmed by immunoblotting using an anti-T7 Ab. C, VHL increases the ubiquitination of myogenin. HEK-293 cells were transfected with His-Ub and T7-Myog and in combination with VHL or VHL C162F. Left, extracts were blotted with an anti-His Ab for the presence of ubiquitinated proteins, anti-T7 Ab for T7-Myog, and anti-HA Ab for VHL or VHL C162F. Right, anti-T7 immunoprecipitates were blotted with an anti-His Ab for the ubiquitinated T7-myogenin and anti-T7 Ab for the precipitated T7-Myog. D, 4.1R reverses the ubiquitination and quantity of myogenin in a VHL-specific manner. HEK-293 cells were transfected with His-Ub, T7-Myog, and either HA-VHL or HA-VHL C162F in the presence or absence of FLAG-4.1R, as indicated. Lysates were detected for the presence of transfected proteins with their respective antibodies. Anti-T7 immunoprecipitates were blotted with an anti-T7 Ab for the presence of T7-Myog. E, effect of proteasome inhibitor MG132 on the ubiquitination status and quantity of myogenin. HEK-293 cells were transfected with His-Ub, T7-Myog, and HA-VHL and either in the presence of FLAG-4.1R or with treatment of the proteasome inhibitor MG132, as indicated. Left, lysates were blotted with an anti-His Ab. The presence of the exogenously expressed proteins was confirmed by immunoblotting with their respective antibodies. Actin served as a loading control. Right, extracts were immunoprecipitated with an anti-T7 Ab and immunoblotted with an anti-His Ab. The presence of T7-Myog was confirmed with an anti-T7 Ab. F, effect of MG132 on myogenin in 4.1R knockdown cells. pTRIPZ4.1R shRNA C2C12 stable lines were cultured in differentiation medium in the presence of doxycycline for 2 days and treated with or without MG132. The amounts of 4.1R and myogenin were determined by immunoblotting with its respective antibodies. β-Actin served as a loading control.