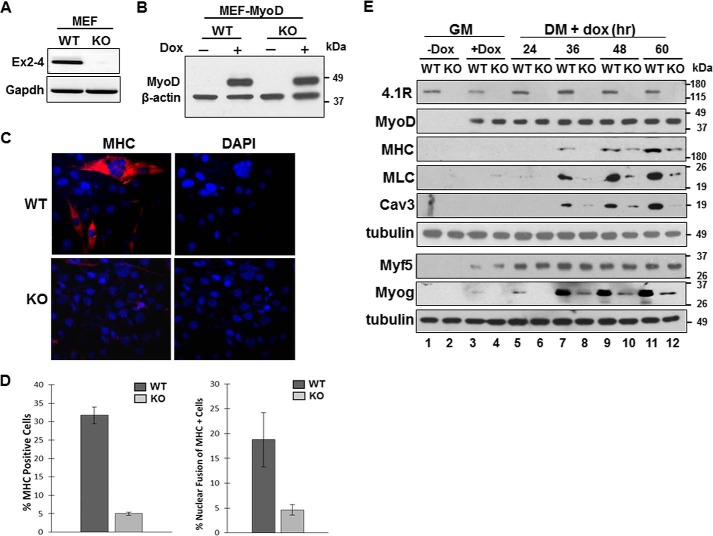
FIGURE 3.
Deletion of 4.1R reduces MyoD-induced differentiation and nuclear fusion in MEFs. A, validation of 4.1R deletion in 4.1R−/− MEF cells. Total RNA isolated from the 4.1R+/+ (WT) and 4.1R−/− (KO) cells were analyzed for the presence of exons 2–4, which contains the translation initiation sites for the 135- and 80-kDa isoforms of 4.1R. GAPDH served as an RT-PCR control. B, WT or KO MEF cells transduced with pTRIPO-3FLAG-MyoD were stably selected with puromycin. Expression of MyoD was analyzed after cells were incubated in differentiation medium in the presence or absence of doxycycline for 24 h. Actin served as a loading control. C, 4.1R deletion reduces myogenic differentiation. 4.1R WT-MyoD and KO-MyoD MEF stable lines were grown in differentiation medium in the presence of doxycycline for 48 h. Differentiation was analyzed by immunofluorescence staining for MHC with an anti-MF20 Ab and Texas Red-conjugated secondary Ab and assessed with Zeiss microscopy (×40). Nuclei were counterstained with DAPI. D, graphical presentation of the effect of 4.1R deletion on differentiation and fusion indices. Left graph, the differentiation indices were calculated as the percentage of MHC-positive cells in each treatment obtained from three independent experiments. Right graph, the fusion indices were calculated as the percentage of cells containing three or more nuclei within the MHC-positive cells. Each experiment was repeated three times, and S.D. values (error bars) were determined. E, 4.1R depletion decreases MHC, MLC, Cav3, and Myog protein expression. WT-MyoD and KO-MyoD MEF cells were grown in growth medium (GM) in the presence or absence of doxycycline for 24 h. Doxycycline-treated MEFs in growth medium were then transited to differentiation medium (DM) in the presence of doxycycline for the indicated periods of time. Cell lysates were collected and immunoblotted for the presence of the indicated protein with its respective Abs. Tubulin served as a loading control.