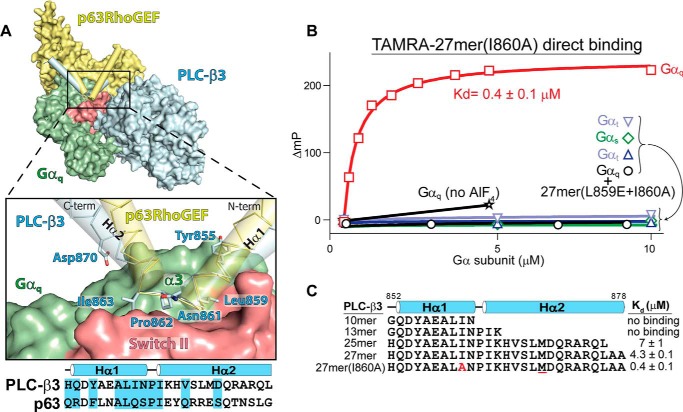
FIGURE 1.
Gαq uses a conserved mechanism to engage effectors. A, a helix-turn-helix (Hα1/Hα2, cylinders) in either PLC-β3 (blue) or p63RhoGEF (yellow) engages a shallow groove on activated Gαq (green with switch regions in red) between switch II and α3. The structures of Gαq bound to either PLC-β3 (PDB code 3OHM) (30) or p63RhoGEF (PDB code 2RGN) (31) were superimposed using Gαq. For clarity, only a single Gαq is shown. The expanded region highlights residues in PLC-β3 that directly contact Gαq; contacts boxed in the corresponding sequence alignment. C-term, C terminus; N-term, N terminus. B and C, peptides derived from the helix-turn-helix of PLC-β3 selectively bind active Gαq. B, fluorescence polarization (ΔmP, change in millipolarization) was used to measure the binding of the indicated Gα subunits to either a TAMRA-labeled peptide (27-mer(I860A)) derived from the helix-turn-helix of PLC-β3 or the equivalent peptide also containing L859E (27-mer(L859E+I860A)). All Gα subunits were activated with aluminum fluoride except where indicated (no AlF4). C, the above assay was used to measure affinities of active Gαq for the TAMRA-labeled peptides listed. Substitutions include I860A (red) and M869Nle (underlined in red).