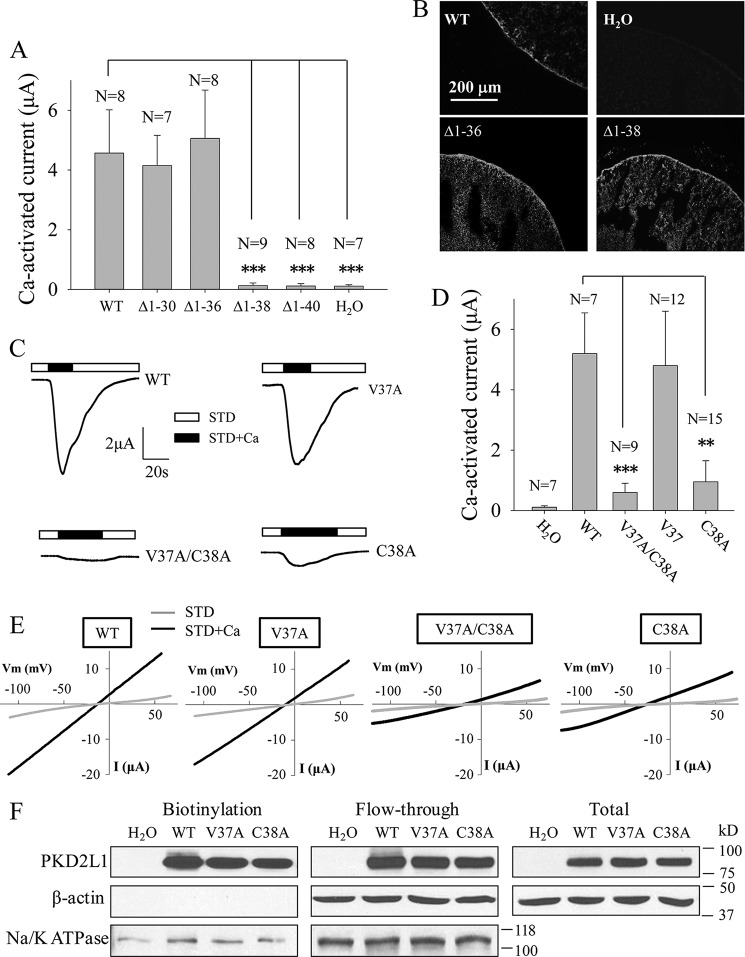
FIGURE 2.
Effects of truncation mutations within Asp-21–Ser-41 and point mutations V37A and C38A on TRPP3 channel function. A, averaged Ca2+-activated currents obtained at −50 mV from oocytes expressing TRPP3 WT or an indicated mutant or from control oocytes (H2O). Currents were averaged from three independent experiments with indicated total numbers of tested oocytes. *** indicates p < 0.001. B, representative immunofluorescence data using oocyte slices, showing expression of TRPP3 WT and indicated truncation mutants. H2O-injected oocytes served as a negative control. C, representative whole-cell current traces obtained from oocytes expressing TRPP3 WT, V37A, C38A, or V37A/C38A (double V37A and C38A mutations) using the TEVC technique under similar experimental conditions as those for Fig. 1C. D, averaged Ca2+-activated currents obtained at −50 mV from oocytes expressing TRPP3 WT, V37A, C38A, or V37A/C38A. Currents were averaged from three independent experiments with indicated total numbers of tested oocytes. ** and *** indicate p < 0.01 and 0.001, respectively. E, representative current-voltage relationship curves obtained using a voltage ramp protocol, as indicated in Fig. 1E, before (STD) and after (STD+Ca) addition of 5 mm CaCl2. F, left panel, representative data on the plasma membrane (PM) expression of TRPP3 by biotinylation. Na+/K+-ATPase (PM marker) and β-actin (non-PM markers) were used as controls. Center and right panels, flow-through and total input data, respectively, from the same experiment.