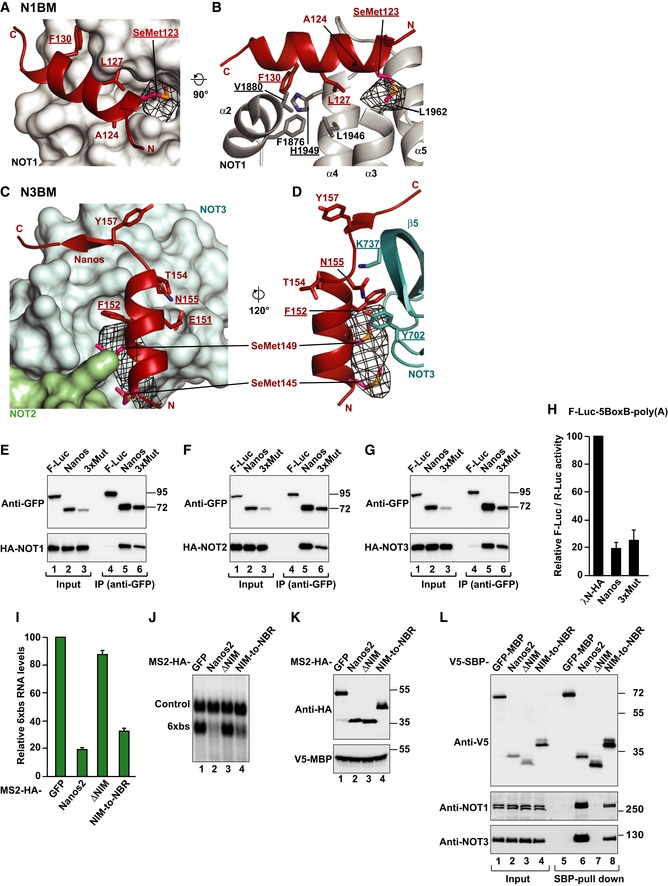
Figure EV5. Validation of the sequence assignment of the NBR peptide bound to the NOT module and activity of NBR mutants.
-
A–DAnomalous difference Fourier map (black mesh) calculated at a resolution of 7.5 Å and contoured at the 4.0 σ level. Data (available as source data) were collected at the Selenium K‐edge peak wavelength from a crystal containing selenomethionine‐substituted Dm Nanos NBR peptide (I123M mutant). The panels show close‐up views of the binding sites of the N1BM (A, B) and the N3BM (C, D) in the same orientations as in Fig 6. Residues mutated in this study are underlined.
-
E–GWestern blot analysis showing the interaction of GFP‐tagged Dm Nanos (wild type or 3xMut) with HA‐tagged NOT1, NOT2, and NOT3. GFP‐tagged firefly luciferase (F‐Luc) served as a negative control. Proteins were immunoprecipitated using a polyclonal anti‐GFP antibody. Inputs and immunoprecipitates were analyzed by Western blotting as described in Fig EV1G–M.
-
HA tethering assay using the F‐Luc‐5BoxB reporter and the indicated λN‐HA‐tagged proteins was performed in S2 as described in Fig 1B. The panel shows mean values ± standard deviations from three independent experiments.
-
ITethering assays in human HEK293T cells, using a β‐globin reporter containing 6 binding sites (6xbs) for the MS2 protein and MS2‐HA‐tagged Hs Nanos2 (wild type or the indicated variants, Nanos2 ΔNIM and Nanos2 NIM‐to‐NBR). In the Nanos2 NIM‐to‐NBR protein, the NIM was replaced by the Dm Nanos NBR. A plasmid expressing an mRNA lacking MS2 binding sites (Control) served as a transfection control. The β‐globin‐6xbs mRNA levels were normalized to those of the control mRNA and set to 100 in the presence of MS2‐HA. The panel shows mean values ± standard deviations from three independent experiments.
-
JNorthern blot of representative RNA samples corresponding to the experiment shown in (I).
-
KWestern blot analysis showing the expression of the MS2‐tagged proteins used in the experiment shown in (I) and (J). V5‐MBP served as a transfection control.
-
LCo‐immunoprecipitation assay in human HEK293T cells showing the interaction of V5‐SBP‐tagged Nanos2 (wild type or the indicated variants) with endogenous NOT1 and NOT3. V5‐SBP‐tagged GFP‐MBP served as a negative control.
Source data are available online for this figure.
