Figure 2.
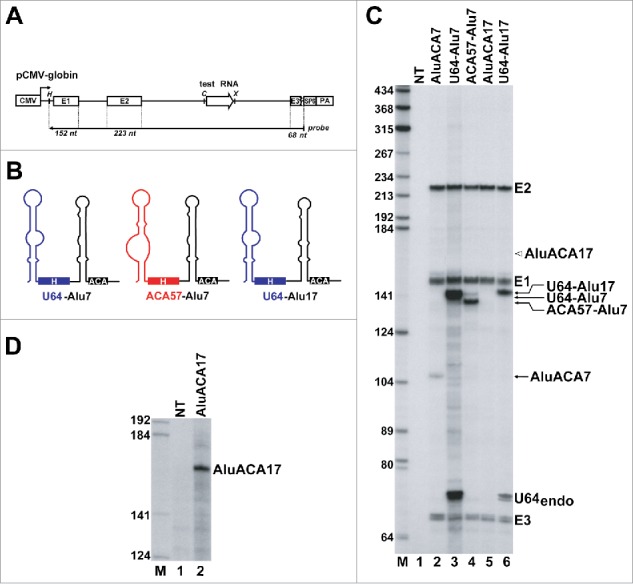
The atypical 5′ hairpins are responsible for inefficient expression of AluACA RNAs. (A) Schematic structure of the pCMV-globin expression vector. The cytomegalovirus (CMV) promoter and polyadenylation site (PA), the β-globin exons (E1, E2 and E3), the intronic test RNA (open arrow) and the SP6 RNA polymerase promoter are shown. The structure of the antisense RNA probe with the expected sizes of the protected fragments are shown. The relevant restriction sites (H, HindIII; C, ClaI; X, XhoI) are indicated. (B) Predicted schematic structures of chimeric H/ACA RNAs. The origin of RNA sequences is indicated by color code (blue, U64 snoRNA; black, AluACA7 and AluACA17; red, ACA57 scaRNA). (C) Processing of transiently expressed globin pre-mRNAs carrying intronic test RNAs. RNAs isolated from HeLa cells non-transfected (NT) or transfected with pCMV-globin expression plasmids carrying the indicated test RNA gene were RNase mapped with sequence-specific antisense RNA probes. The protected probe RNA fragments were separated on a 6% sequencing gel. Bands corresponding to the spliced globin exons (E1, E2 and E3) and the processed test RNAs are indicated. The fragment U64endo was protected by the HeLa endogenous U64 snoRNA and its identity was confirmed by control mappings (data not shown). Open arrow indicates the expected position of AluACA17. Lane M, DNA size markers in nucleotides. (D) Detection of transiently over-expressed AluACA17 RNA. Total RNA from HeLa cells transfected with the pCMV-globin-AluACA17 expression plasmid were mapped with a high specific activity antisense RNA probe complementary to AluACA17. Please note that under the applied mapping conditions, HeLa endogenous AluACA7 (panel C, lane 1) and AluACA17 (panel D, lane 1) remained indiscernible.
