Figure 4.
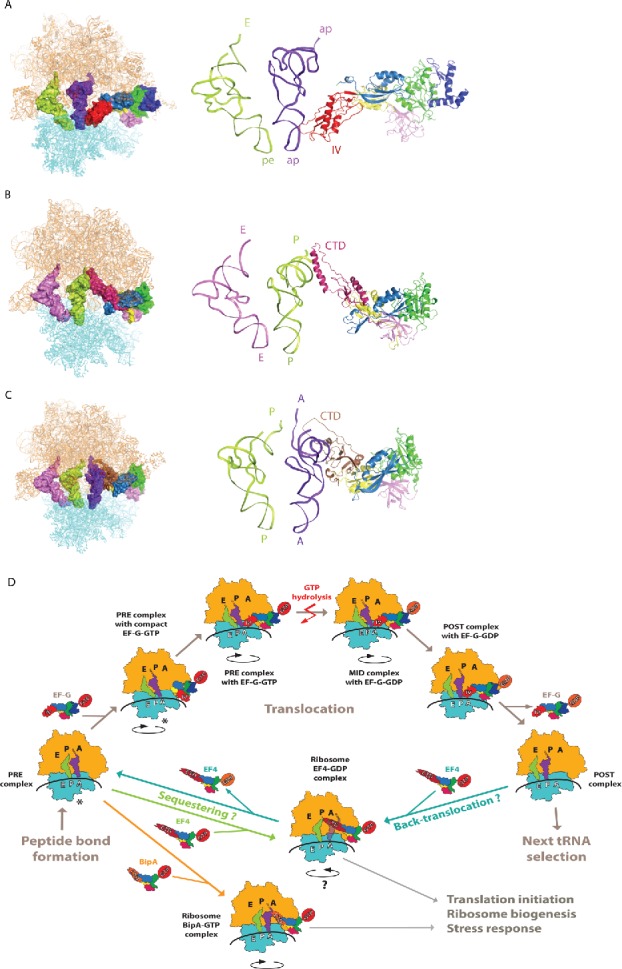
Structural insight into EF-G, EF4, and BipA functioning in translation. (A) Interaction of EF-G domain IV with the ASL of tRNA as revealed by the mid-translocation (MID) complex structure75 shown as surface on the ribosome (left) and as a cartoon in close-up (right). (B) Interaction of EF4 CTD with the P site tRNA in PTC region.47 (C) Interaction of BipA CTD with the A site tRNA in PTC region.46 trGTPases are colored as previously, ribosome 30S and 50S subunits are colored cyan and orange, respectively. tRNAs are colored based on the positioning of their ASL with respect to the decoding center on 30S. (D) Schematic representation of EF-G, EF4, and BipA functioning in translation based on recent structural studies. In short, after peptide bond formation, EF-G recognizes the PRE translocation complex. EF-G first interacts with the ribosome in its compact conformation. Conformational changes in EF-G lead to the transition into elongated form with domain IV extending toward the decoding center in A site coupled to the stabilization of the anti-clockwise rotation of the 30S subunit. Following GTP hydrolysis, the interaction between domain IV of EF-G and the ASL of A site tRNA is likely maintained as the tRNAs-mRNA complex is shifted one codon relative to the ribosome and the 30S subunit returns to the unrotated state. GDP form EF-G dissociates from the POST translocation leaving it ready for next cycle of translation elongation. Asterisk highlights that the PRE complex fluctuates between unrotated and rotated state, both of which are recognized by EF-G and observed in complex with compact EF-G. Under stress conditions, EF4 interacts with either POST “mis-translocation” complex and reverts it back to PRE state allowing EF-G another chance at correct translocation. Alternatively, EF4 as well as BipA can compete with EF-G for the PRE complex and regulate translation in response to stress by likely affecting co-translational protein folding or translation of specific proteins.
