Abstract
Many neuropsychiatric and neurodevelopmental disorders such as schizophrenia and autism involve interneuron transcriptional dysregulation. The transcriptional coactivator PGC-1α regulates gene expression in GABAergic interneurons, which are important for regulating hippocampal network activity. Genetic deletion of PGC-1α causes a decrease in parvalbumin expression, similar to what is observed in schizophrenia postmortem tissue. Our lab has previously shown that PGC-1α−/− mice have enhanced GABAergic inhibition onto CA1 pyramidal cells, which increases the inhibition/excitation (I/E) ratio, alters hippocampal circuit function, and impairs hippocampal dependent behavior. The typical antipsychotic haloperidol, a dopamine receptor antagonist with selectivity for D2-like receptors, has previously been shown to increase excitation in the CA1 region of hippocampus. We therefore tested whether haloperidol could normalize the I/E balance in CA1 of PGC-1α−/− mice, potentially improving circuit function and behavior. Surprisingly, we discovered instead that interneuron transcriptional dysregulation caused by loss of PGC-1α alters the effects of haloperidol on hippocampal synaptic transmission and circuit function. Acute administration of haloperidol causes disinhibition in CA1 and decreases the I/E ratio onto CA1 pyramidal cells in slices from PGC-1α+/+ mice, but not PGC-1α−/− mice. The spread of activity in CA1, assessed by voltage sensitive dye imaging, is increased by haloperidol in slices from PGC-1α+/+ mice; however haloperidol decreases the spread of activity in slices from PGC-1α−/− mice. Haloperidol increased the power of hippocampal gamma oscillation in slices from PGC-1α+/+ mice but reduced the power of gamma oscillations in slices from PGC-1α−/− mice. Nest construction, an innate hippocampal-dependent behavior, is inhibited by haloperidol in PGC-1α+/+ mice, but not in PGC-1α−/− mice, which already have impaired nest building. The effects of haloperidol are mimicked and occluded by a D2 receptor antagonist in slices from PGC-1α+/+ mice, and the effects of blocking D2 receptors are lost in slices from PGC-1α−/− mice, although there is no change in D2 receptor transcript levels. Together, our results show that hippocampal inhibitory synaptic transmission, CA1 circuit function, and hippocampal dependent behavior are modulated by the antipsychotic haloperidol, and that these effects of haloperidol are lost in PGC-1α−/− mice. These results have implications for the treatment of individuals with conditions involving PGC-1α deficiency.
1. Introduction
Transcriptional dysregulation in inhibitory interneurons is seen in a variety of neuropsychiatric and neurodevelopmental disorders, including schizophrenia and autism (Lewis and Hashimoto, 2007). Decreased expression of the calcium binding protein parvalbumin, which is found primarily in fast-spiking interneurons, is consistently reported from studies on postmortem tissue from schizophrenia patients (Blum and Mann, 2002; Eyles et al., 2002; Knable et al., 2004; Reynolds et al., 2001, 2004). Parvalbumin levels are also reduced in autism (Gandal et al., 2012) and bipolar disorder (Sibille et al., 2011). However, our understanding of the effects of interneuron transcriptional dysregulation and parvalbumin reduction on synaptic and circuit function is incomplete.
PGC-1α (peroxisome proliferator activated receptor γ co-activator 1α) is a transcriptional coactivator that in hippocampus is concentrated in GABAergic interneurons. PGC-1α regulates the expression of parvalbumin (Lucas et al., 2010), and deletion of PGC-1α causes reductions in parvalbumin and other proteins that regulate GABA release, including synaptotagmin 2 and complexin 1 (Bartley et al., 2015; Lucas et al., 2010). PGC-1α−/− mice provide a way to investigate the effects of interneuron transcriptional dysregulation on synaptic and circuit function. Our lab has previously shown that interneuron transcriptional dysregulation caused by loss of PGC-1α causes a frequency-dependent increase in inhibitory synaptic transmission onto CA1 pyramidal cells (Bartley et al., 2015). This increase in the I/E ratio greatly reduces the spread of activation in CA1 and impairs spiking of CA1 pyramidal cells, reducing hippocampal output (Bartley et al., 2015). PGC-1α−/− mice also have enhanced power of gamma oscillations and an impairment in nest building (Bartley et al., 2015), an innate hippocampal-dependent behavioral task (Deacon, 2006), consistent with impaired hippocampal circuit function (Bartley et al., 2015).
Haloperidol, a dopamine receptor antagonist with selectivity for dopamine D2-like receptors, is an antipsychotic used in schizophrenia patients to treat positive symptoms that arise from enhanced dopamine signaling (Davis et al., 1991). Although hippocampal dysfunction contributes to cognitive impairment in schizophrenia (Gastambide et al., 2015), and dopamine receptors regulate inhibition in hippocampus (Gonzalez-Islas and Hablitz, 2001), haloperidol has not been found to be effective for treating the cognitive symptoms in schizophrenia (Furth et al., 2013). It is currently not known how haloperidol will affect hippocampal circuit function altered as a result of interneuron transcriptional dysregulation.
Previously it has been shown that acute application of haloperidol to hippocampal slices from wildtype mice causes an enhancement of excitatory synaptic transmission in CA1 (Baskys et al., 1993), suggesting that haloperidol could alleviate the I/E imbalance in CA1 caused by loss of PGC-1α. Here we compared the effects of haloperidol on synaptic transmission, circuit function, and behavior in PGC-1α−/− and PGC-1α+/+ mice. We find that acute application of haloperidol reduces feed-forward inhibition and the I/E ratio in CA1 pyramidal cells in slices from PGC-1α+/+ mice, and enhances the spread of activation in CA1 as measured by voltage-sensitive dye imaging. Surprisingly, none of these effects were observed in slices from PGC-1α−/− mice, indicating that the ability of haloperidol to cause disinhibition is lost in these mice. Furthermore, haloperidol increased the power of gamma oscillations in PGC-1α+/+ slices, but reduced gamma power in PGC-1α−/− slices. Haloperidol significantly impaired nest construction by PGC-1α+/+ mice, however haloperidol caused no further effect on nest building behavior in PGC-1α−/− mice, which was already impaired. Blocking D2 receptors with a specific antagonist mimicked and occluded the effects of haloperidol in slices from PGC-1α+/+ mice but had no effect in slices from PGC-1α−/− mice, confirming the effects of haloperidol are through D2 receptors. Together, our results show that transcriptional dysregulation in interneurons alters the modulation of inhibition and hippocampal circuit function by the antipsychotic haloperidol.
2. Materials and Methods
2.1 Animals
All experimental procedures for the care and use of laboratory animals were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee, and were conducted in accordance with the U.S. National Institute of Health Guide for the Care and Use of Laboratory Animals. Male and female PGC-1α+/+ and PGC-1α−/− mice generated from PGC-1α+/− breeding pairs were used. Mice were maintained on a C57BL/6J genetic background. Mice were housed in cages at 80 °F with food and water ad libitum. Mouse genotypes were determined from tail biopsies using real time PCR with specific probes designed for PGC-1α (Transnetyx, Cordova, TN).
2.2 Slice preparation
Male and female mice, postnatal (P) day 25 to P60, were anesthetized with isoflurane and sacrificed by decapitation using a rodent guillotine. The brains were rapidly removed and placed in ice cold dissection solution containing the following: 120 NaCl, 3.5 KCl, 0.75 CaCl2, 4.0 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose, bubbled with 95% O2/5% CO2, pH 7.35–7.45, and osmolarity 295–305. 400 µm thick coronal slices (or 300 µm thick horizontal slices for gamma oscillation experiments) of the hippocampus were cut on a vibrating microtome (VT1000S; Leica, Bannockburn, IL) in ice cold dissection solution. Slices were stored in a recovery chamber containing room temperature dissection solution bubbled with 95% O2/5% CO2 for at least 1 hour before recording, except slices for gamma oscillation experiments, which were incubated in dissection solution at 32 °C for 1 hour before being stored at room temperature. Slices used for experiments with inhibition blocked had area CA3 removed during slice preparation.
2.3 Electrophysiology
2.3.1 Field Potential Recordings
Extracellular field potential recordings from acute ventral hippocampal slices from PGC-1α+/+ and PGC-1α−/− mice were performed at 30 °C in a submerged recording chamber on a Nikon (New York, NY) Optiphot-2. The slices wereperfused with external recording solution (ERS) containing the following: 120 NaCl, 3.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose, bubbled with 95% O2/5% CO2, pH 7.35–7.5, osmolarity 295–305. Field postsynaptic potentials (fPSPs) were recorded using an ERS-filled microelectrode in response to extracellular stimulation of Schaffer collateral axons in the stratum radiatum layer of CA1 with a bipolar tungsten microelectrode (FHC, Bowdoinham, ME). In experiments where inhibition was blocked the ERS contained 100 µM picrotoxin (Abcam, Cambridge, MA) to block GABAA receptors. Haloperidol and L-741,626 were obtained from Abcam.
2.3.2 Whole-cell Recordings
Whole-cell patch-clamp recordings were acquired by blindly patching CA1 pyramidal cells in the voltage-clamp configuration on a Nikon (New York, NY) Eclipse E600-FN upright microscope. Patch electrodes were filled with internal solution composed of the following (in mM): 125 Cs-Gluconate, 0.6 EGTA, 1.0 MgCl2, 3 MgSO4, 25 HEPES, 10 Na-ATP, 0.3 GTP, 5 phospocreatine, pH was adjusted to 7.2 with CsOH. The internal solution also contained 10 mM Cs-BAPTA to block depolarization-induced suppression of inhibition (Lenz and Alger, 1999), and 2 mM QX-314 (N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium chloride) to improve space clamp and reduce nonlinear effects caused by voltage-gated channels in dendrites while recording from the pyramidal cell soma. For these experiments ERS also contained 50 µM D-APV (D-2-amino-5-phosphonopentanoic acid) to block NMDA receptor-mediated currents and prevent postsynaptic short-term plasticity, as well as to prevent long-term potentiation and long-term depression; 1 µM AM 251 (N-(Piperidin-1-yl)-5-(4-iodophenyl)--1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-car-boxamide) to block CB1 receptors and prevent depolarization-induced suppression of inhibition (De-May and Ali, 2013); and 10 µM CGP 55845 ((2S)-3-[[(1S)-1-(3,4-Dichlorophenyl-)ethyl]amino-2-hydroxypropyl](phenylmethyl) phosphinic acid) to block GABAB receptors, including the receptors localized presynaptically at Schaffer collateral synapses, thereby preventing activity dependent reduction in glutamate release (Speed and Dobrunz, 2008). APV, CGP 55845, and AM251 have previously been shown to have no effect on the I/E ratio induced by paired-pulse stimulation (Bartley and Dobrunz, 2015). To measure excitation, inhibition, and the I/E ratio, CA1 pyramidal cells were held at the reversal potentials for excitation (0 mV) and inhibition (−55 mV) to measure EPSCs and disynaptic feed-forward IPSCs, respectively, in response to Schaffer collateral axon stimulation. In our previous study (Bartley et al., 2015), no difference was observed in the EPSC amplitude between PGC-1α+/+ and PGC-1α−/− mice at any given stimulus intensity. Therefore, the stimulus intensity was set to obtain an EPSC between 125 to 200 pA in both genotypes. There was no difference in the stimulus intensity used (PGC-1α+/+ (100.43 ± 23.34) µA vs PGC-1α−/− (99.55 ± 17.91) µA; Student’s t-test, p>0.05). The I/E ratio was calculated from the peaks of the EPSCs and IPSCs (Bartley and Dobrunz, 2015; Bartley et al., 2015; Torborg et al., 2010). For analysis of disynaptic IPSC kinetics, the half-width of the response was measured. Monosynaptic IPSCs were measured by holding CA1 pyramidal cells at 0 mV and stimulating inhibitory interneuron axons in stratum radiatum, with excitatory synapses blocked by NBQX (10 µM) and D-AP5 (50 µM).
2.3.3 Gamma Oscillation Recordings
Local field potential recordings from stratum pyramidale of CA3 in horizontal hippocampal slices (300 µm) were performed at 32 °C in a dual-submerged recording chamber which perfuses the slice from above and below (RC-27L, Warner Instruments, CT, USA). 100 nM kainate was applied to generate gamma oscillations (Fuchs et al., 2007). Fast Fourier transforms for power spectra were computed from 1 s traces using Visual Basic software, and power values were obtained by integrating power spectra between 20 and 80 Hz.
2.4 Voltage Sensitive Dye Imaging
The spatiotemporal spread of activity in CA1 in response to Schaffer collateral axon stimulation was measured using voltage-sensitive dye (VSD) imaging as previously described (Bartley et al., 2015; Calfa et al., 2011). Slices recovered for at least an hour in dissection solution, then were transferred to ERS with the addition of the voltage-sensitive dye, RH-414. The dye RH-414 (30 µM) was applied for at least 45 minutes before imaging. The amplitude, spatial spread, and duration were measured in response to single-pulse stimulation of Schaffer collateral axons in slices from PGC-1α+/+ and PGC-1α−/− mice before and during acute application of haloperidol (1 µM).
2.5 Nesting Behavior
Nesting behavior was tested in individually housed PGC-1α+/+ and PGC-1α−/− male and female mice, age P55–P96, as described previously (Bartley et al., 2015), and scored based on a 5 point system (Bartley et al., 2015; Deacon, 2012). The first nest score was measured without application of any drug. The second nest score was obtained after application of vehicle (0.9% saline, 1 mL/ 100 g body weight) or haloperidol (1 mg/kg) (Xu et al., 2012) by intraperitoneal (i.p.) injection. I.p. injections were administered approximately 1 hour before the addition of the nestlet (3g; Ancare). The next morning nests were inspected, pictures were taken for documentation, and the untorn nestlet was weighed. Animals with evidence of motor impairment after administration of haloperidol (1/13 PGC-1α+/+, 2/11 PGC-1α−/−) were not included in the analysis.
2.6 Gene expression analyses
Mice were anesthetized with isoflurane and decapitated. Whole hippocampi were dissected, frozen on dry ice, and stored at −80 °C to await RNA isolation, DNase pretreatment, reverse transcription, and Taqman q-RT-PCR as described in (Dougherty et al., 2014; Lucas et al., 2010). Applied Biosystems inventoried primer/probe sets for PGC-1α (Mm00447183_m1), parvalbumin (Mm0044310_m1), complexin-1 (Mm00514378_m1), synaptotagmin-2 (Mm00436864_m1), and DRD2 (Hs00241436_m1) were used. Relative concentrations of the genes of interest were calculated in comparison to a standard curve calculated from dilutions of cDNA (calibrator method; 1:5, 1:10, 1:20, 1:40) from a pool of littermate control cDNA. Values were normalized to β-actin (Mm00607939_s1) and expressed as arbitrary units ± standard error (SE).
2.7 Statistical analysis
All statistics were performed using Origin software (Origin Lab Corporation, 2002) and statistical significance was p<0.05. Data are presented as means ± SE and sample number (n) refers to cell (whole-cell) or slice number for electrophysiological and VSD experiments, and number of animals for behavioral experiments. Statistical comparisons were made using the Student’s t-test, one-way ANOVA, and two-way ANOVA, followed by Tukey’s posthoc analysis as indicated in figures and in text.
3. Results
3.1 Haloperidol causes disinhibition in slices from PGC-1α+/+ mice but not PGC-1α−/− mice
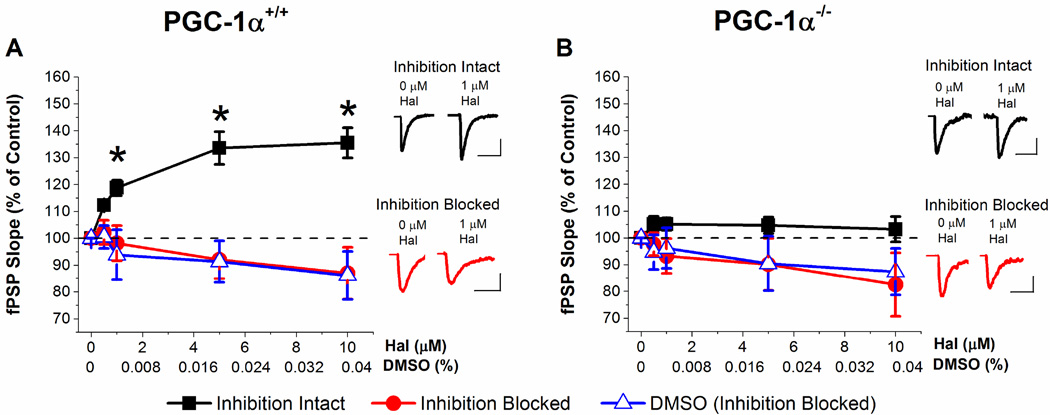
We first compared the effects of haloperidol on field potential recordings from the Schaffer collateral-CA1 pathway in acute slices from PGC-1α−/− and PGC-1α+/+ mice. Figure 1A shows that acute application of haloperidol potentiates the fPSP in a dose-dependent manner in slices from PGC-1α+/+ mice when inhibition is intact in conjunction with previous observations (Baskys et al., 1993). Haloperidol had little to no effect on the fPSP when inhibition was blocked. This indicates that the effect of haloperidol to increase the fPSP is caused by disinhibition, rather than by an increase in excitation. In contrast, haloperidol has no significant effect on the fPSP in slices from PGC-1α−/− mice, either with inhibition intact or with inhibition blocked (Figure 1B). The slight decrease in the synaptic response with inhibition blocked in both PGC-1α+/+ and PGC-1α−/− mice is due to using DMSO as a solvent for haloperidol (Figure 1A, B). DMSO alone was bath applied to slices to control for its potential effects revealing a small decrease in synaptic transmission (Figure 1A, B). This is consistent with reports of side effects of DMSO on synaptic activity (Lu and Mattson, 2001; Sawada and Sato, 1975; Tsvyetlynska et al., 2005). These results suggest that the disinhibitory effect of haloperidol on the Schaffer collateral-CA1 pathway is lost in slices from PGC-1α−/− mice.
Figure 1. Haloperidol increases the fPSP in slices from PGC-1α+/+ mice but not PGC-1α−/− mice.
A. Dose response curve of fPSPs after application of increasing concentrations of haloperidol (with inhibition intact or inhibition blocked by 100 µM picrotoxin) or DMSO (with inhibition blocked) in PGC-1α+/+. Inhibition intact (n=8, two way ANOVA, F(6,89) = 15.02, * = p < 0.05), inhibition blocked (n=6), DMSO (n=6). Insets: Example traces from inhibition intact (black) and inhibition blocked (red) hippocampal slices at 0 µM haloperidol (left) and1 µM haloperidol (right) concentration. B. Dose response curve of fPSPs after application of increasing concentrations of haloperidol (with inhibition intact or inhibition blocked) or DMSO (with inhibition blocked) in PGC-1α−/−. Insets: Example traces from inhibition intact (black) and inhibition blocked (red) hippocampal slices at 0 µM haloperidol (left) and1 µM haloperidol (right) concentration. Inhibition intact (n=8), inhibition blocked (n=7), DMSO (n=6). Scale Bars: 20 ms, 0.3 mV (inhibition intact and Inhibition blocked: PGC-1α+/+ and PGC-1α−/−).
3.2 Haloperidol decreases inhibition and I/E ratio in slices from PGC-1α+/+ mice but not PGC-1α−/− mice
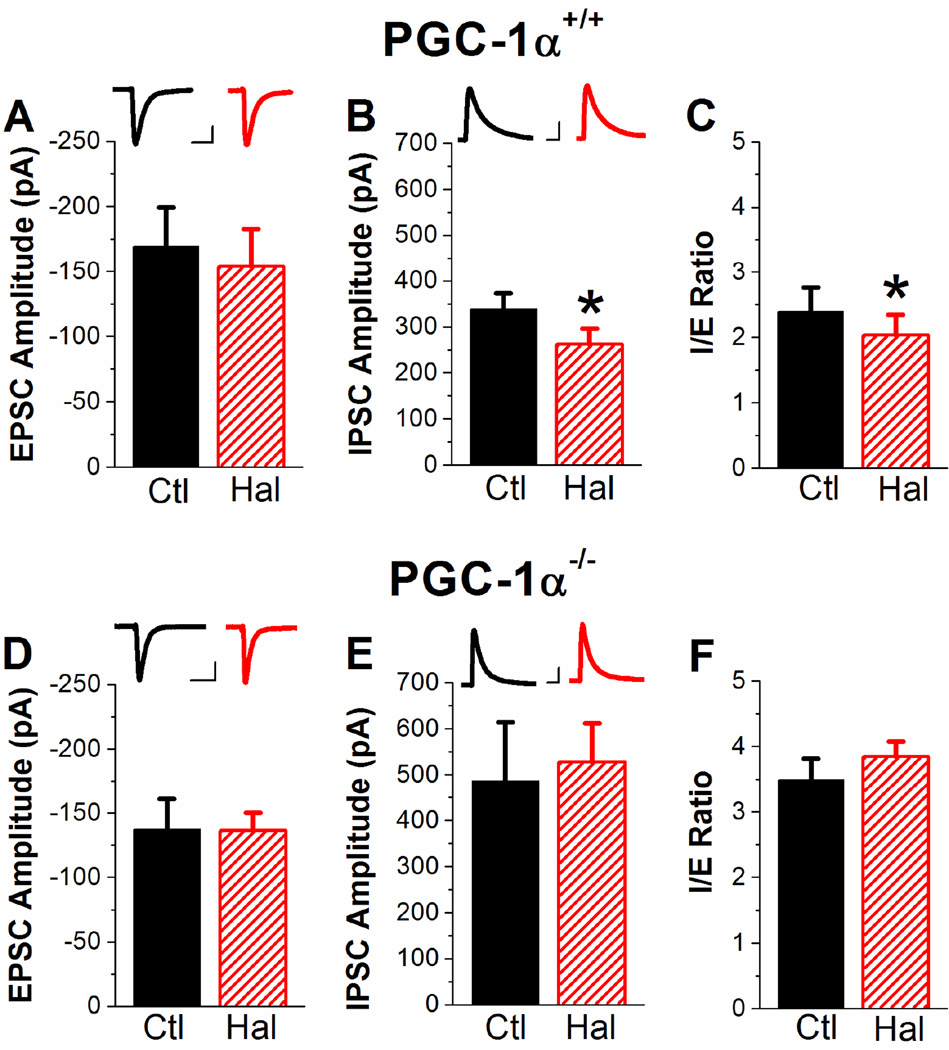
Field potential recordings show the effects of populations of excitatory and inhibitory synapses in area CA1 of the hippocampus, but are not a direct measurement of inhibition. We next directly tested the effects of haloperidol on inhibition and excitation using whole-cell voltage clamp recordings from CA1 pyramidal cells in PGC-1α+/+ and PGC-1α−/− slices. In slices from PGC-1α+/+ mice, there is no significant effect of 1 µM haloperidol on the EPSC amplitude (Figure 2A). However, haloperidol significantly decreases the IPSC amplitude (Figure 2B), resulting in a decrease in the I/E ratio (Figure 2C). In PGC-1α−/− slices, haloperidol has no significant effect on either excitation (Figure 2D) or inhibition (Figure 2E), and does not alter the I/E ratio (Figure 2F). Haloperidol did not alter the kinetics of the IPSCs for either genotype (two way ANOVA, F(1,33)=0.26, P=0.61). However, the IPSCs are narrower in the PGC-1α−/− slices (PGC-1α+/+ 60.5 ± 4.7 ms vs. PGC-1α−/− 37.6 ± 3.6 ms; two way ANOVA, F(1,33)=23.81, p<0.05). Together, these results confirm that haloperidol has a disinhibitory effect on synaptic transmission in CA1 in slices from PGC-1α+/+ mice, and that this effect is lost in slices from PGC-1α−/− mice. This suggests that transcriptional dysregulation in PGC-1α−/− mice causes an impairment in the regulation of inhibition and the I/E ratio by haloperidol. The results also show that the I/E ratio is greater in slices from PGC-1α−/− mice compared to PGC-1α+/+ mice as a result of enhanced inhibition, as we have previously shown (Bartley et al., 2015).
Figure 2. Haloperidol reduces inhibition and I/E ratio in slices from PGC-1α+/+ mice but not PGC-1α−/− mice.
A–C. Quantification of haloperidol effects on EPSC amplitude (A, n=7, two-way ANOVA, F(2,25)=1.0, p=0.38), IPSC amplitude (B, n=11, two-way ANOVA, F(2,33)=19.7, * = p<0.05), and I/E ratio (C, n=7, two-way ANOVA, F(2,25)=14.0, * = p<0.05) in PGC-1α+/+ slices. Scale Bars: 40 ms, 50 pA (A), 40 ms, 100 pA (B). D–F. Quantification of haloperidol effects on EPSC amplitude (D, n=7), IPSC amplitude (E, n=7), and I/E ratio (F, n=7) in PGC-1α−/− slices. Scale Bars: 40 ms, 50 pA (D), 40 ms, 100 pA (E).
3.3 Voltage-sensitive dye imaging shows haloperidol increases the spread of activity in CA1 in slices from PGC-1α+/+ mice but decreases spread of activity in slices from PGC-1α−/− mice
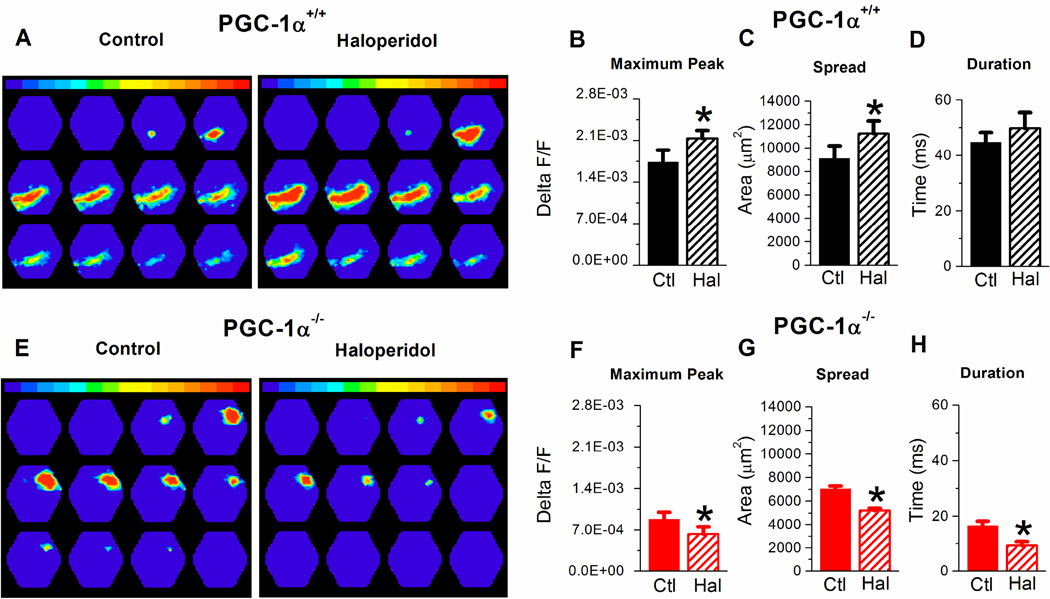
The enhanced I/E ratio in PGC-1α−/− mice has been shown to cause a reduction in the spread of activation in hippocampal CA1, as measured with voltage-sensitive dye (VSD) imaging (Bartley et al., 2015). We next compared the effect of haloperidol on the spread of activation in CA1 in slices from PGC-1α+/+ and PGC-1α−/− mice (Figure 3). Figure 3A shows examples of the time sequence of VSD images, separated by 2.49 ms, from a slice from a PGC-1α+/+ mouse before and during application of 1 µM haloperidol. In slices from PGC-1α+/+ mice, acute application of haloperidol increases the activation in CA1 in response to Schaffer collateral stimulation, consistent with its effect to cause disinhibition. The maximum peak of the response (Figure 3B) and spatial spread of activation (Figure 3C) are significantly increased by haloperidol in slices from PGC-1α+/+ mice. There was a trend toward an increase in the duration of the response with application of haloperidol in slices from PGC-1α+/+ mice, although not significant (Figure 3D). However, the effect of haloperidol was different in slices from PGC-1α−/− mice. Example images (Figure 3E) show that haloperidol instead decreases the spread of activation in CA1 in a slice from a PGC-1α−/− mouse. There is a significant reduction in the maximum peak, spatial spread of activation and the duration of the response with haloperidol application (Figure 3F–H). This shows that the normal effect of haloperidol on circuit function is lost in slices from PGC-1α−/− mice, and it instead appears to make the circuit dysfunction in PGC-1α−/− slices worse.
Figure 3. Voltage-sensitive dye imaging shows haloperidol increases the spread of activity in CA1 in slices from PGC-1α+/+ mice but decreases spread of activity in slices from PGC-1α−/− mice.
A. Example image time sequence of the spatiotemporal pattern of VSD signals in CA1 from PGC-1α+/+ slice (left) and PGC-1α+/+ slice treated with 1 µM haloperidol (right) in response to single pulse stimulation of SC axons at 70 µA. Each frame is 2.49 ms apart. B–D. Quantification of haloperidol effects on maximum peak (B, n=7, two-way ANOVA, F(3,28)=23.1, * = p<0.05), spread (C, (n=7, two-way ANOVA, F(3,28)=14.3, * = p<0.05), and duration (D, n=7) in PGC-1α+/+ slices. E. Example image time sequence of the spatiotemporal pattern of VSD signals evoked in CA1 from PGC-1α−/− slice (left) and PGC-1α−/−slice treated with 1 µM haloperidol (right) in response to single pulse stimulation of SC axons at 70 µA. F–H. Quantification of haloperidol effects on maximum peak (F, n=9, *=p<0.05), spread (G, n=9, *=p<0.05), and duration (H, n=9, two-way ANOVA, F(3,28)=40.3, * = p<0.05) in PGC-1α−/− slices.
3.4 Haloperidol increases the power of hippocampal gamma oscillations in slices from PGC-1α+/+ mice but reduces the power of gamma oscillations in slices from PGC-1α−/− mice
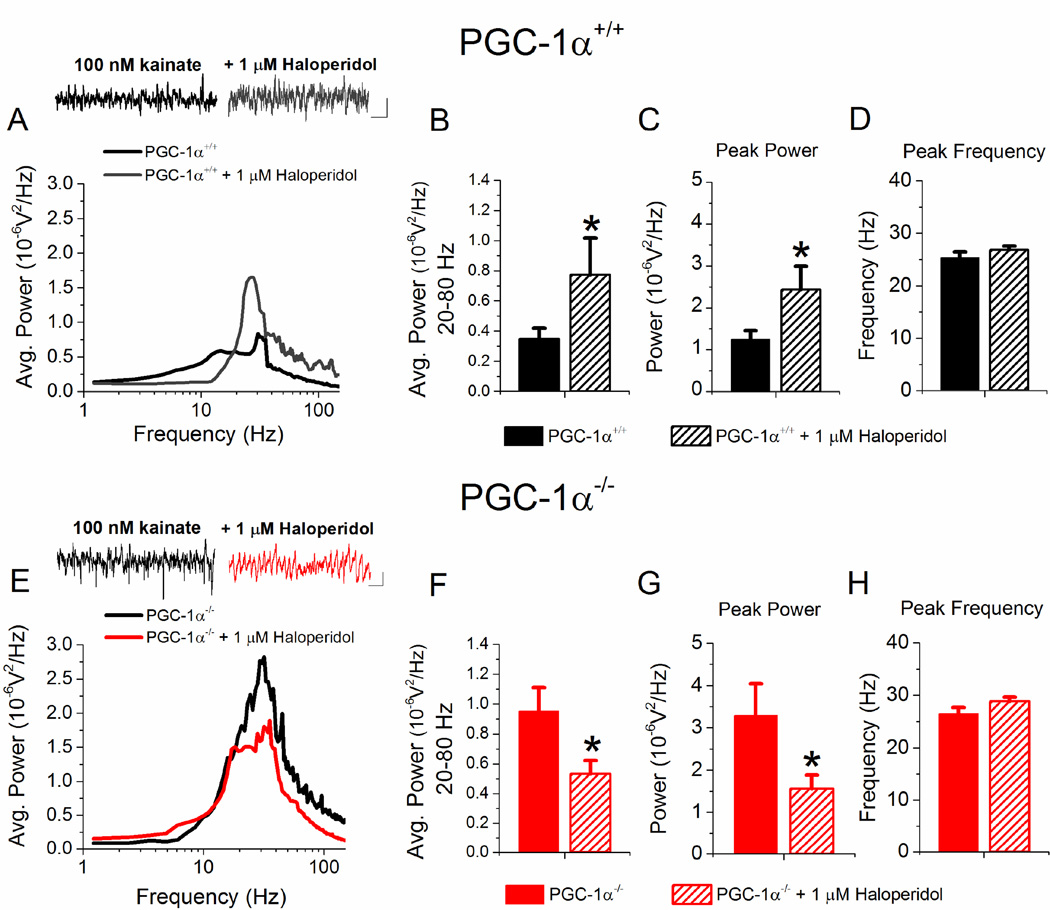
Another measure of hippocampal circuit function is gamma oscillations, which can be induced in hippocampal slices by bath application of kainate (Tsintsadze et al., 2015). We have previously shown that the power of hippocampal gamma oscillations is increased in slices from PGC-1α−/− mice compared to PGC-1α+/+ mice (Bartley et al., 2015). Here we tested the effects of haloperidol, and found that acute administration of 1 µM haloperidol to PGC-1α+/+ slices causes a significant increase in the average power of oscillations between 20–80 Hz (Figure 4A and 4B) as well as an increase in the peak power (Figure 4C), with no change in the peak frequency (Figure 4D). However, the effect of haloperidol was different in slices from PGC-1α−/− mice, where haloperidol instead significantly reduced gamma oscillation power (Figure 4E and 4F) as well as the peak power (Figure 4G). Again, there was no change in peak frequency (Figure 4H). These results show that haloperidol has opposite effects on gamma oscillations based on genotype.
Figure 4. Haloperidol increases gamma oscillation power in slices from PGC-1α+/+ mice but reduces the power of gamma oscillations in PGC-1α−/− mice.
A. Average power spectrum and example traces from PGC-1α+/+ slices (black, n=19) and PGC-1α+/+ + 1 µM Haloperidol (gray, n=11) after application of 100 nM kainate to induce oscillations in the gamma frequency range (20–80 Hz). Scale Bar: 100 ms, 0.02 mV. B–D. Quantification of the effects of 1 µM Haloperidol on the average power in the gamma frequency range (B, n=11, two way ANOVA, F(2,58) = 12.52, * = p < 0.05), average peak power (C, n=11, two way ANOVA, F(2,58) = 12.12, * = p < 0.05), and average peak frequency (D) in PGC-1α+/+ slices. E. Average power spectrum and example traces from PGC-1α−/− control (black, n=18) and PGC-1α−/− + 1 µM Haloperidol (red, n=17) after application of 100 nM kainate to induce oscillations in the gamma frequency range (20–80 Hz). Scale Bar: 100 ms, 0.02 mV. F–H. Quantification of the effects of 1 µM Haloperidol on the average normalized power in the gamma frequency range (F, n =17 two-way ANOVA, F(2,58) = 13.36, * = p < 0.05), average peak power (G, n=17, two-way ANOVA, F(2,58) = 15.78, * = p < 0.05), and average peak frequency (H) in PGC-1α−/− slices.
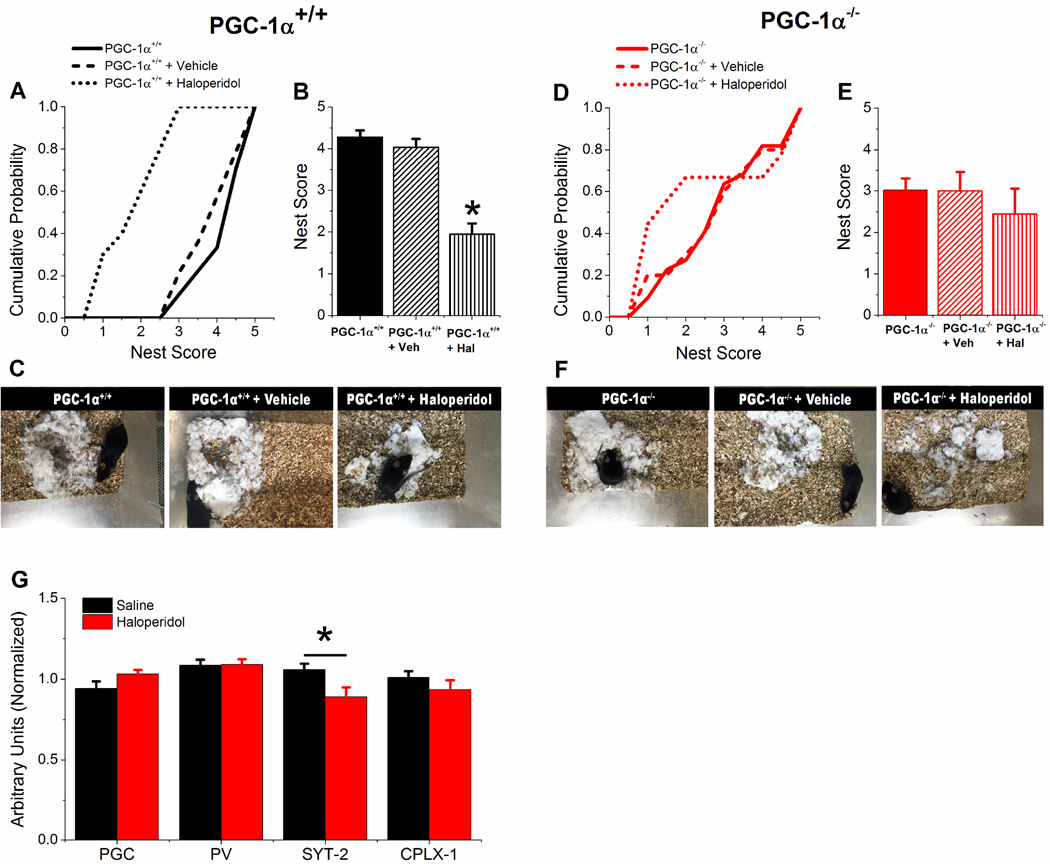
3.5 Haloperidol impairs nest construction of PGC-1α+/+ mice but not PGC-1α−/− mice
Hippocampal damage and malfunction in mice have been shown to impair nest building, an innate hippocampus-dependent non-learned behavior (Jirkof, 2014; Kaur et al., 2014). Previously we have shown that PGC-1α−/− mice had a lower score in nest construction compared to PGC-1α+/+ mice (Bartley et al., 2015). Here we tested the effects on nest construction of a single dose of haloperidol (1 mg/kg) administered by intraperitoneal injection to PGC-1α+/+ and PGC-1α−/− mice (Figure 5). Haloperidol injection in PGC-1α+/+ mice causes a significant decrease in the scores for nest building (Figure 5A and 5C), however the injection of haloperidol does not affect nest scores in PGC-1α−/− mice (Figure 5B and 5D), which are already reduced compared to PGC-1α+/+ mice. Acute injection of haloperidol also did not affect transcript levels for PGC-1α, parvalbumin, or complexin-1 levels in hippocampus of PGC-1α+/+ mice (Figure 5G). There was, however, a statistically significant decrease in synaptotagmin-2 levels after injection of haloperidol, which could potentially play a role in haloperidol’s effects in PGC-1α+/+ mice. The fact that there is no consistent effect on PGC-1α target genes in haloperidol-treated PGC-1α+/+ mice suggests that haloperidol is exerting its effects through a PGC-1α - independent mechanism. However, it is still possible that post-translational modifications of PGC-1α (Scarpulla, 2011) are occurring that influence the expression of PGC-1α targets that were not tested in this study. Unbiased transcriptomic profiling of hippocampal neuronal populations in PGC-1α+/+ haloperidol-treated mice would begin to address this issue. Overall, these data show that haloperidol has an inhibitory effect on the natural hippocampal-dependent behavior of nest construction in PGC-1α+/+ mice, and that the effect is lost in PGC-1α−/− mice, showing that transcriptional dysregulation in interneurons alters the behavioral responses to haloperidol.
Figure 5. Haloperidol decreases nesting behavior of PGC-1α+/+ mice but not PGC-1α−/− mice.
A. Cumulative probability of nest scores of PGC-1α+/+ mice before (n=24) and after i.p. injection of vehicle (saline, n=14) or haloperidol (n=10). B. Average nest scores of PGC-1α+/+ mice before and after i.p. injection of vehicle or haloperidol (n = 10, two-way ANOVA, F(1,21) = 10.10, * = p< 0.05). C. Example images of nest construction for PGC-1α+/+ mice before and after vehicle and haloperidol i.p. injections. D. Cumulative probability of nest scores of PGC-1α−/− mice before (n=18) and after i.p. injection of vehicle (saline, n=8) or haloperidol (n=10). E. Average nest scores of PGC-1α−/− mice and after i.p. injection of vehicle or haloperidol. F. Example images of nest construction for PGC-1α−/− mice before and after vehicle and haloperidol i.p. injections. G. q-RT-PCR results showing effect of saline and haloperidol i.p. injections into PGC-1α+/+ mice (n= 12, 11) on transcript expression of PGC-1α (PGC), parvalbumin (PV), synaptotagmin-2 (SYT-2), and complexin-1 (CPLX-1) in hippocampal homogenates, normalized to β-actin. Student’s t-test, * = p < 0.05.
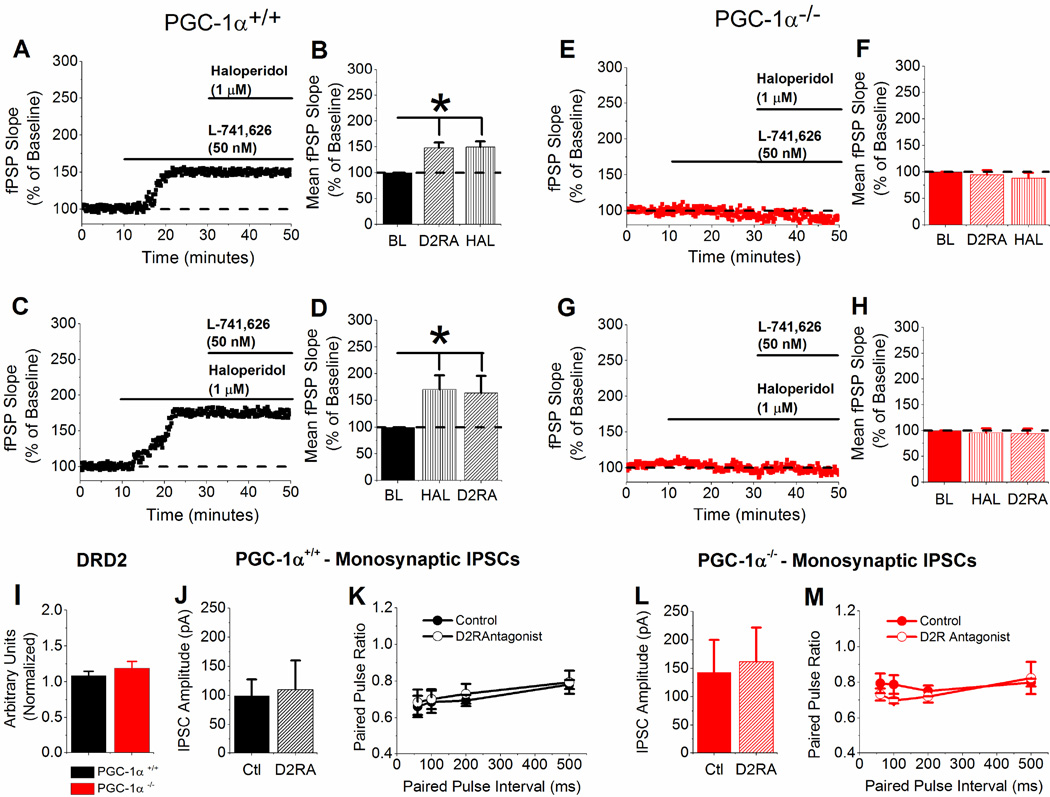
3.6 Dopamine D2 receptor antagonist mimics and occludes the effects of haloperidol in PGC-1α+/+ slices and has no effect in slices from PGC-1α−/− mice
To rule out nonspecific effects of haloperidol, we measured the effects of a highly specific dopamine D2 receptor antagonist on the synaptic response in both PGC-1α+/+ and PGC-1α−/− slices. L-741,626 (50 nM) mimicked the effects of haloperidol in PGC-1α+/+ slices but had no effect in PGC-1α−/− slices, and the subsequent addition of 1 µM haloperidol had no additional effect on the synaptic response for either genotype (Figure 6A, B, E, and F). Furthermore, bath application of haloperidol occluded the effects of L-741,626 in PGC-1α+/+ slices and had no effect in PGC-1α−/− (Figure 6C, D, G, and H). There was no effect of blocking D2 receptors on monosynaptic IPSCs onto CA1 pyramidal cells and the paired pulse ratio in either PGC-1α+/+ slices or PGC-1α−/− slices (Figure 6J – M). Surprisingly there was no difference in DRD2 transcript levels in PGC-1α−/− mice (Figure 6I), which suggests that the amount of dopamine D2 receptors in PGC-1α−/− mice is not changed. Together, these results suggest that haloperidol is exerting its effects through dopamine D2 receptors and that interneuron transcriptional dysregulation caused by genetic deletion of PGC-1α blocks the effects of D2 receptor activation on synaptic function.
Figure 6. L-741,626 mimics and occludes the effects of haloperidol in PGC-1α+/+ slices and has no effect in slices from PGC-1α−/− mice.
A – B. Example experiment and group data of application of L-741,626 (50 nM), then Haloperidol (1 µM) on fPSP slope (B, n=6, one-way ANOVA, F(2,15) = 10.16 * = p <0.05) in PGC-1α+/+ slices. C – D. Example experiment and group data of application of Haloperidol (1 µM), then L-741,626 (50 nM) on fPSP slope (D, n=5, one-way ANOVA, F(2,12) = 10.1 * = p <0.05) in PGC-1α+/+ slices. E – F. Example experiment and group data of application of L-741,626 (50 nM), then Haloperidol (1 µM) on fPSP slope (B, n=7) in PGC-1α−/− slices. G – H. Example experiment and group data application of Haloperidol (1 µM), then L-741,626 (50 nM) on fPSP slope (D, n=7) in PGC-1α−/− slices. I. q-RT-PCR results of DRD2, normalized to β-actin, from PGC-1α+/+ and PGC-1α−/− hippocampal homogenates. J – K. Quantification of evoked monosynaptic IPSC amplitude (J) and paired pulse ratio (K) in PGC-1α+/+ slices (n=6) with and without application of 50 nM L-741,626. L – M. Quantification of evoked monosynaptic IPSC amplitude (L) and paired pulse ratio (M) in PGC-1α−/− slices (n=4) with and without application of 50 nM L-741,626.
3.7 Summary
Transcriptional dysregulation in interneurons caused by deletion of PGC-1α causes significant changes in hippocampal synaptic transmission, circuit function and behavior (Bartley et al. 2015, summarized in Table 2). In this study we show that most of the observed effects of haloperidol on synaptic transmission, circuit function, and behavior in PGC-1α+/+ mice are not seen in PGC-1α−/− (Table 1), indicating that transcriptional dysregulation in interneurons modifies the modulation of synaptic transmission, circuit function and behavior by haloperidol. To determine whether haloperidol normalized any of the changes in synaptic transmission, circuit function and behavior in PGC-1α−/− mice to PGC-1α+/+ levels, we compared PGC-1α−/− + Haloperidol to PGC-1α+/+ alone (Table 2). This comparison revealed that haloperidol normalized the power of hippocampal gamma oscillations in PGC-1α−/− slices to PGC-1α+/+ levels (ANOVA, F(1,22) = 1.14, 20–80 Hz average power: p = 0.298, F(1,34) = 0.69, peak power: p = 0.411). This was the only aspect of hippocampal synaptic function or behavior that was rescued by haloperidol. These data suggest that the differential effects of haloperidol on circuit function helps to normalize the difference in gamma oscillation power caused by interneuron transcriptional dysregulation in slices from PGC-1α+/+ mice.
Table 2.
Haloperidol rescues only gamma oscillations in PGC-1α−/−
| PGC-1α−/− control vs PGC-1α+/+ control |
Haloperidol rescues PGC-1α−/− |
|
|---|---|---|
| I/E ratio | ↑ | No |
| Disynaptic Inhibition |
↑ | No |
| Monosynaptic Inhibition |
↑ | No |
| VSD Activity | ↓ | No |
| Gamma Oscillations |
↑ | Yes |
| Nesting Behavior | ↓ | No |
↑ = Significantly greater ↓ = Significantly reduced
Comparison of PGC-1α+/+ and PGC-1α−/− slices/mice for the indicated parameters in the left column. The far right column is a comparison of effects between between PGC-1α+/+ control and PGC-1α−/− slices/mice + haloperidol or the specific D2 receptor antagonist (L-741,626) to determine if application of haloperidol restored effects in PGC-1α−/− back to PGC-1α+/+ levels.
Table 1.
Effects of haloperidol on hippocampal synaptic transmission and behavior
| PGC-1α+/+ Hal (or D2RA) vs control |
PGC-1α−/− Hal (or D2RA) vs control |
|
|---|---|---|
| I/E ratio | ↓ | ↔ |
| Disynaptic Inhibition |
↓ | ↔ |
| Monosynaptic Inhibition |
↔ | ↔ |
| EPSC | ↔ | ↔ |
| VSD Activity | ↑ | ↓ |
| Gamma Oscillations |
↑ | ↓ |
| Nesting Behavior | ↓ | ↔ |
↑ = Significant increase ↓ = Significant decrease ↔ = No Change
Summary of effects of haloperidol or the specific D2 receptor antagonist (L-741,626) compared to control for PGC-1α+/+ and PGC-1α−/−.
4. Discussion
The purpose of the present investigation was to test whether haloperidol could normalize the I/E imbalance in PGC-1α−/− mice caused by interneuron transcriptional dysregulation. Instead, we discovered that interneuron transcriptional dysregulation alters the effects of haloperidol on hippocampal synaptic transmission and circuit function. Our results indicate that the normal disinhibitory effect of haloperidol on synaptic transmission in CA1 is lost in PGC-1α−/− slices, as is its ability to increase CA1 activation, measured by VSD imaging. In addition, the effect of haloperidol on kainate-induced gamma oscillations is altered in PGC-1α−/− mice; haloperidol increases gamma power in slices from PGC-1α+/+ mice but decreases gamma power in PGC-1α−/− mice. Finally, haloperidol causes impaired nest building in PGC-1α+/+ mice, an effect that is also lost in PGC-1α−/− mice. Together, these results show that transcriptional dysregulation in interneurons can alter the hippocampal synaptic, circuit, and behavioral responses to a typical antipsychotic drug.
The search for drug targets that restore I/E balance is important because of the multitude of evidence showing that alterations in GABAergic neurotransmission cause disturbed I/E balance and result in changes to brain dynamics (Uhlhaas and Singer, 2012). Our lab has previously shown that PGC-1α−/− mice have an enhanced I/E ratio, caused by an increase in disynaptic inhibition (Bartley et al., 2015). This causes reduced hippocampal output, which is similar in some respects to the hippocampal hypofunction seen in the neonatal ventral hippocampal lesion model of schizophrenia (O`Donnell, 2012). In hippocampus, haloperidol had previously been shown to increase fPSPs in CA1, an effect that was thought to be caused by enhanced excitation (Baskys et al., 1993). Consistent with this, we find that acute application of haloperidol decreases the I/E ratio in slices from PGC-1α+/+ mice. However, we find that the effect of haloperidol is caused by disinhibition, rather than a direct effect on excitation. In contrast, haloperidol no longer reduces inhibition, and the I/E ratio is unaffected by haloperidol, in slices from PGC-1α−/− mice. As a result, haloperidol is unable to rescue the I/E imbalance in these mice. The loss of the disinhibitory effect of haloperidol in PGC-1α−/− slices indicates that transcriptional dysregulation in interneurons can alter the effects of antipsychotic drugs on inhibitory synaptic transmission.
Haloperidol is an antagonist with high affinity for dopamine D2 receptors (Davis et al., 1991). Although haloperidol can have effects at other receptors, we show that specifically blocking D2 receptors mimics and occludes haloperidol’s effect in slices from PGC-1α+/+ mice. D2 receptors in hippocampus are located on GABAergic interneurons (Puighermanal et al., 2015), consistent with the effects of haloperidol to modulate disynaptic inhibition but not excitation. Although presynaptic D2 receptors have been shown to modulate GABA release at inhibitory synapses onto pyramidal cells in amygdala (Chu et al., 2012), we saw no effect of blocking D2 receptors on monosynaptic inhibition onto CA1 pyramidal cells. D2 receptors must therefore affect the recruitment of interneurons, by modulating their intrinsic excitability (Cazorla et al., 2012; Tseng and O`Donnell, 2007), and/or their inputs (Tritsch and Sabatini, 2012). Because the effects of haloperidol in PGC-1α+/+ mice are through inhibitory transmission, our data suggest that the regulation of inhibition by the dopamine system, and in particular D2 receptors, is altered in hippocampus of PGC-1α−/− mice. Similarly, a loss of D2 receptor modulation of inhibition is also seen in prefrontal cortex in the neonatal ventral hippocampal lesion model (Tseng et al., 2008). The loss of D2 modulation could be caused by a reduction in receptors (Lucas et al., 2012) or through alterations in downstream signaling (Tritsch and Sabatini, 2012). Because there was no change in hippocampal D2 receptor transcripts from PGC-1α−/− mice, the effect is most likely from alterations in D2 signaling mechanisms. However, alterations in the regulation of inhibition by D2 receptors are not the cause of the I/E imbalance in PGC-1α−/− mice, because blocking D2 receptors with haloperidol does not rescue the imbalance.
Impaired neural synchrony has been demonstrated in many disorders with neuropsychiatric and neurodevelopmental origin. Increased gamma activity has been observed in autism (Dickinson et al., 2015), epilepsy (Stroganova et al., 2015), ADHD (Karch et al., 2012), bipolar disorder (Brealy et al., 2015), and in positive symptoms of schizophrenia (Herrmann and Demiralp, 2005). Haloperidol significantly reduces the power of baseline EEG gamma oscillations in wild type rats (Jones et al., 2012), suggesting that it may be beneficial in diseases with increased gamma oscillations. PGC-1α−/− mice have an increase in hippocampal gamma power (Bartley et al., 2015), which could be caused by their parvalbumin deficiency, as seen in parvalbumin−/− mice (Vreugdenhil et al., 2003). Haloperidol reduced the power of gamma oscillations in slices from PGC-1α−/−, eliminating the disparity between the two genotypes. This was the only aspect of synaptic function or behavior that was rescued by haloperidol. The effect of haloperidol on gamma oscillations is different from its effect on inhibition, suggesting that the increased inhibition is likely not the cause of the increased gamma oscillations in PGC-1α−/− slices. It is possible that haloperidol’s effect on gamma oscillations are through serotonin receptors (Bombardi and Di Giovanni, 2013; Schulz et al., 2012), rather than dopamine receptors. It remains to be determined whether haloperidol also has differential effects on gamma oscillations in vivo in mice with transcriptional dysregulation.
Our results show that nest building is impaired both in the PGC-1α−/− mice, which have an enhanced I/E ratio, and in PGC-1α+/+ mice with haloperidol, which have a reduced I/E ratio. This suggests that deviation of the I/E ratio from the normal range in either direction is detrimental to hippocampal circuit function and behavior. Consistent with this, impaired nest building has been seen in both a mouse model related to autism spectrum disorder (Moretti et al., 2005), which has enhanced excitation (Moretti et al., 2006), and in a Down Syndrome mouse model (Heller et al., 2014) which has enhanced inhibition (Kleschevnikov et al., 2004). Furthermore, the dissociation between the effects of haloperidol on gamma oscillations and nest building in the PGC-1α−/− mice suggests that the alterations in gamma oscillations are not likely to underlie the behavioral effects on nest building.
Previous studies have shown that loss of PGC-1α has opposite effects on inhibition in cortex (Dougherty et al., 2014) as compared to hippocampus (Bartley et al., 2015; Lucas et al., 2012). It remains to be determined whether the effects of haloperidol on synaptic and circuit function are also altered in the cortex of PGC-1α−/− mice. Consistent with the idea that haloperidol has region-specific effects, a single dose of haloperidol has been shown to increase or decrease activity in schizophrenia patients in different brain regions (Lahti et al., 2005). Region-specific differences such as these may explain why some medications are effective for symptoms originating in certain parts of the brain but are ineffective or even detrimental on symptoms originating from circuit dysfunction in other areas.
While we only examined acute effects of haloperidol in this study, chronic treatment with haloperidol has been shown to also have effects on gene expression (Carboni and Domenici, 2016). If these changes in gene expression are triggered by haloperidol’s effects on D2 receptors, they may also be lost or altered in in PGC-1α−/− mice. Long-term use of antipsychotic drugs has been shown to regulate the expression of GABAergic genes implicated in neuropsychiatric disorders including schizophrenia (Peselmann et al., 2013). This means that antipsychotic application not only alters circuit function through the dopaminergic and/or serotinergic systems, but could alter circuit function by changing inhibitory synaptic transmission. Furthermore, these effects could potentially be different in diseases with a transcriptional dysregulation component.
Cognitive enhancement is a treatment goal for a variety of neuropsychiatric and neurological illnesses. Even though dopamine D2 receptors play a major role in cognition and mood regulation, blockade by antipsychotic drugs, such as haloperidol, often fail to alleviate cognitive dysfunction. Our study in wildtype mice suggests that haloperidol could improve certain aspects of cognitive function, since excitation was increased through disinhibition and gamma oscillations were enhanced, often associated with improved performance on memory related tasks (Lewis et al., 2008). However, in our animal model of interneuron transcriptional dysregulation haloperidol had no effect, and in some cases it worsened the phenotype. Our results suggest that the ineffectiveness of haloperidol to alleviate cognitive dysfunction could be due to the alterations in interneuron function and expression of key proteins. Since transcriptional dysregulation in interneurons has been observed in a wide range of neurodevelopmental and neuropsychiatric disorders, medications that improve cognition in a control population could be completely ineffective for patients with neurological diseases. Therefore, it is important to test cognitive enhancers in a disease model with impairments in cognition.
Highlights.
Haloperidol causes disinhibition in hippocampus mediated by dopamine D2 receptors
This decreases the I/E ratio and enhances circuit activity but impairs behavior
Haloperidol’s effects are lost in PGC-1α−/− mice, and circuit function is altered
Haloperidol restores γ oscillation power in PGC-1α−/− slices, which was enhanced
Transcriptional dysregulation alters haloperidol’s effects on hippocampal function
Acknowledgments
This study was supported by National Institutes of Health grants R01MH098534 to L.E.D., R01NS070009 to R.M.C., and P30NS047466. L.J.B. was supported by training grants T32GM008111 and T32NS061788.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartley AF, Dobrunz LE. Short-term plasticity regulates the excitation/inhibition ratio and the temporal window for spike integration in CA1 pyramidal cells. Eur J Neurosci. 2015;41:1402–1415. doi: 10.1111/ejn.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley AF, Lucas EK, Brady LJ, Li Q, Hablitz JJ, Cowell RM, Dobrunz LE. Interneuron Transcriptional Dysregulation Causes Frequency-Dependent Alterations in the Balance of Inhibition and Excitation in Hippocampus. J Neurosci. 2015;35:15276–15290. doi: 10.1523/JNEUROSCI.1834-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskys A, Wang S, Remington G, Wojtowicz JM. Haloperidol and loxapine but not clozapine increase synaptic responses in the hippocampus. Eur J Pharmacol. 1993;235:305–307. doi: 10.1016/0014-2999(93)90151-7. [DOI] [PubMed] [Google Scholar]
- Blum BP, Mann JJ. The GABAergic system in schizophrenia. Int J Neuropsychopharmacol. 2002;5:159–179. doi: 10.1017/S1461145702002894. [DOI] [PubMed] [Google Scholar]
- Bombardi C, Di Giovanni G. Functional anatomy of 5-HT2A receptors in the amygdala and hippocampal complex: relevance to memory functions. Exp Brain Res. 2013;230:427–439. doi: 10.1007/s00221-013-3512-6. [DOI] [PubMed] [Google Scholar]
- Brealy JA, Shaw A, Richardson H, Singh KD, Muthukumaraswamy SD, Keedwell PA. Increased visual gamma power in schizoaffective bipolar disorder. Psychol Med. 2015;45:783–794. doi: 10.1017/S0033291714001846. [DOI] [PubMed] [Google Scholar]
- Calfa G, Hablitz JJ, Pozzo-Miller L. Network hyperexcitability in hippocampal slices from Mecp2 mutant mice revealed by voltage-sensitive dye imaging. J Neurophysiol. 2011;105:1768–1784. doi: 10.1152/jn.00800.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni L, Domenici E. Proteome effects of antipsychotic drugs: Learning from preclinical models. Proteomics Clin Appl. 2016;10:430–441. doi: 10.1002/prca.201500087. [DOI] [PubMed] [Google Scholar]
- Cazorla M, Shegda M, Ramesh B, Harrison NL, Kellendonk C. Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J Neurosci. 2012;32:2398–2409. doi: 10.1523/JNEUROSCI.6056-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HY, Ito W, Li J, Morozov A. Target-specific suppression of GABA release from parvalbumin interneurons in the basolateral amygdala by dopamine. J Neurosci. 2012;32:14815–14820. doi: 10.1523/JNEUROSCI.2997-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Deacon R. Assessing burrowing, nest construction, and hoarding in mice. J Vis Exp. 2012:e2607. doi: 10.3791/2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- De-May CL, Ali AB. Cell type-specific regulation of inhibition via cannabinoid type 1 receptors in rat neocortex. J Neurophysiol. 2013;109:216–224. doi: 10.1152/jn.00272.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Bruyns-Haylett M, Jones M, Milne E. Increased peak gamma frequency in individuals with higher levels of autistic traits. Eur J Neurosci. 2015;41:1095–1101. doi: 10.1111/ejn.12881. [DOI] [PubMed] [Google Scholar]
- Dougherty SE, Bartley AF, Lucas EK, Hablitz JJ, Dobrunz LE, Cowell RM. Mice lacking the transcriptional coactivator PGC-1a exhibit alterations in inhibitory synaptic transmission in the motor cortex. Neuroscience. 2014;271:137–148. doi: 10.1016/j.neuroscience.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles DW, McGrath JJ, Reynolds GP. Neuronal calcium-binding proteins and schizophrenia. Schizophr Res. 2002;57:27–34. doi: 10.1016/s0920-9964(01)00299-7. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Furth KE, Mastwal S, Wang KH, Buonanno A, Vullhorst D. Dopamine, cognitive function, and gamma oscillations: role of D4 receptors. Front Cell Neurosci. 2013;7:102. doi: 10.3389/fncel.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Nesbitt AM, McCurdy RM, Alter MD. Measuring the maturity of the fast-spiking interneuron transcriptional program in autism, schizophrenia, and bipolar disorder. PLoS ONE. 2012;7:e41215. doi: 10.1371/journal.pone.0041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastambide F, Taylor AM, Palmer C, Svard H, Karjalainen M, Janhunen SK, Tricklebank M, Bannerman DM. Alterations in spatial memory and anxiety in the MAM E17 rat model of hippocampal pathology in schizophrenia. Psychopharmacology (Berl) 2015;232:4099–4112. doi: 10.1007/s00213-014-3862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J Neurophysiol. 2001;86:2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- Heller HC, Salehi A, Chuluun B, Das D, Lin B, Moghadam S, Garner CC, Colas D. Nest building is impaired in the Ts65Dn mouse model of Down syndrome and rescued by blocking 5HT2a receptors. Neurobiol Learn Mem. 2014;116:162–171. doi: 10.1016/j.nlm.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Jirkof P. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods. 2014;234:139–146. doi: 10.1016/j.jneumeth.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Jones NC, Reddy M, Anderson P, Salzberg MR, O`Brien TJ, Pinault D. Acute administration of typical and atypical antipsychotics reduces EEG γ power, but only the preclinical compound LY379268 reduces the ketamine-induced rise in γ power. Int J Neuropsychopharmacol. 2012;15:657–668. doi: 10.1017/S1461145711000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch S, Segmiller F, Hantschk I, Cerovecki A, Opgen-Rhein M, Hock B, Dargel S, Leicht G, Hennig-Fast K, Riedel M, et al. Increased γ oscillations during voluntary selection processes in adult patients with attention deficit/hyperactivity disorder. J Psychiatr Res. 2012;46:1515–1523. doi: 10.1016/j.jpsychires.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Kaur G, Sharma A, Xu W, Gerum S, Alldred MJ, Subbanna S, Basavarajappa BS, Pawlik M, Ohno M, Ginsberg SD, et al. Glutamatergic transmission aberration: a major cause of behavioral deficits in a murine model of Down`s syndrome. J Neurosci. 2014;34:5099–5106. doi: 10.1523/JNEUROSCI.5338-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Villar AJ, Epstein CJ, Malenka RC, Mobley WC. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J Neurosci. 2004;24:8153–8160. doi: 10.1523/JNEUROSCI.1766-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9:609–620. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Medoff DR, Tamminga CA, Holcomb HH. Functional effects of single dose first- and second-generation antipsychotic administration in subjects with schizophrenia. Psychiatry Res. 2005;139:19–30. doi: 10.1016/j.pscychresns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lenz RA, Alger BE. Calcium dependence of depolarization-induced suppression of inhibition in rat hippocampal CA1 pyramidal neurons. J Physiol (Lond) 1999;521(Pt 1):147–157. doi: 10.1111/j.1469-7793.1999.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T. Deciphering the disease process of schizophrenia: the contribution of cortical GABA neurons. Int Rev Neurobiol. 2007;78:109–131. doi: 10.1016/S0074-7742(06)78004-7. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Mattson MP. Dimethyl sulfoxide suppresses NMDA- and AMPA-induced ion currents and calcium influx and protects against excitotoxic death in hippocampal neurons. Exp Neurol. 2001;170:180–185. doi: 10.1006/exnr.2001.7686. [DOI] [PubMed] [Google Scholar]
- Lucas EK, Markwardt SJ, Gupta S, Meador-Woodruff JH, Lin JD, Overstreet-Wadiche L, Cowell RM. Parvalbumin deficiency and GABAergic dysfunction in mice lacking PGC-1alpha. J Neurosci. 2010;30:7227–7235. doi: 10.1523/JNEUROSCI.0698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas EK, Dougherty SE, McMeekin LJ, Trinh AT, Reid CS, Cowell RM. Developmental alterations in motor coordination and medium spiny neuron markers in mice lacking pgc-1a. PLoS ONE. 2012;7:e42878. doi: 10.1371/journal.pone.0042878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O`Donnell P. Cortical disinhibition in the neonatal ventral hippocampal lesion model of schizophrenia: new vistas on possible therapeutic approaches. Pharmacol Ther. 2012;133:19–25. doi: 10.1016/j.pharmthera.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Peselmann N, Schmitt A, Gebicke-Haerter PJ, Zink M. Aripiprazole differentially regulates the expression of Gad67 and γ-aminobutyric acid transporters in rat brain. Eur Arch Psychiatry Clin Neurosci. 2013;263:285–297. doi: 10.1007/s00406-012-0367-y. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Biever A, Espallergues J, Gangarossa G, De Bundel D, Valjent E. drd2-cre:ribotag mouse line unravels the possible diversity of dopamine d2 receptor-expressing cells of the dorsal mouse hippocampus. Hippocampus. 2015;25:858–875. doi: 10.1002/hipo.22408. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Zhang ZJ, Beasley CL. Neurochemical correlates of cortical GABAergic deficits in schizophrenia: selective losses of calcium binding protein immunoreactivity. Brain Res Bull. 2001;55:579–584. doi: 10.1016/s0361-9230(01)00526-3. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Abdul-Monim Z, Neill JC, Zhang ZJ. Calcium binding protein markers of GABA deficits in schizophrenia--postmortem studies and animal models. Neurotox Res. 2004;6:57–61. doi: 10.1007/BF03033297. [DOI] [PubMed] [Google Scholar]
- Sawada M, Sato M. The effect of dimethyl sulfoxide on the neuronal excitability and cholinergic transmission in Aplysia ganglion cells. Ann N Y Acad Sci. 1975;243:337–357. doi: 10.1111/j.1749-6632.1975.tb25375.x. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz SB, Heidmann KE, Mike A, Klaft ZJ, Heinemann U, Gerevich Z. First and second generation antipsychotics influence hippocampal gamma oscillations by interactions with 5-HT3 and D3 receptors. Br J Pharmacol. 2012;167:1480–1491. doi: 10.1111/j.1476-5381.2012.02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed HE, Dobrunz LE. Developmental decrease in short-term facilitation at Schaffer collateral synapses in hippocampus is mGluR1 sensitive. J Neurophysiol. 2008;99:799–813. doi: 10.1152/jn.00625.2007. [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Butorina AV, Sysoeva OV, Prokofyev AO, Nikolaeva AY, Tsetlin MM, Orekhova EV. Altered modulation of gamma oscillation frequency by speed of visual motion in children with autism spectrum disorders. J Neurodev Disord. 2015;7:21. doi: 10.1186/s11689-015-9121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Nakashiba T, Tonegawa S, McBain CJ. Control of CA3 output by feedforward inhibition despite developmental changes in the excitation-inhibition balance. J Neurosci. 2010;30:15628–15637. doi: 10.1523/JNEUROSCI.3099-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O`Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61:843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O`Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsintsadze V, Minlebaev M, Suchkov D, Cunningham MO, Khazipov R. Ontogeny of kainate-induced gamma oscillations in the rat CA3 hippocampus in vitro. Front Cell Neurosci. 2015;9:195. doi: 10.3389/fncel.2015.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvyetlynska NA, Hill RH, Grillner S. Role of AMPA receptor desensitization and the side effects of a DMSO vehicle on reticulospinal EPSPs and locomotor activity. J Neurophysiol. 2005;94:3951–3960. doi: 10.1152/jn.00201.2005. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89:1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- Xu H, Yang HJ, Rose GM. Chronic haloperidol-induced spatial memory deficits accompany the upregulation of D(1) and D(2) receptors in the caudate putamen of C57BL/6 mouse. Life Sci. 2012;91:322–328. doi: 10.1016/j.lfs.2012.07.025. [DOI] [PubMed] [Google Scholar]