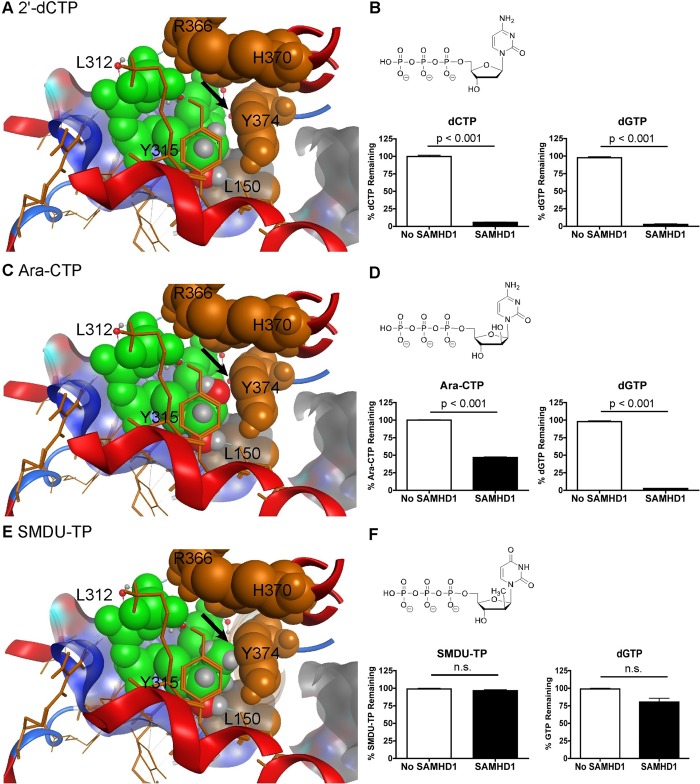
Fig 4. Role of Y374 and C2' sugar moiety substitution in acting as substrates of SAMHD1.
A) dCTP, C) ara-CTP and E) SMDU-TP nucleotides (in green) are modeled within the catalytic site of SAMHD1. Both dCTP and ara-CTP do not clash with Y374 (see arrow). However the model shows that the (2'S)-2'-methyl group of SMDU-TP clashes with Y374 in the catalytic pocket of SAMHD1. B, D and F) Determining if dCTP, ara-CTP and SMDU-TP can be hydrolyzed for SAMHD1 in vitro. Structures of the compounds are above the HLPC graphs with experimental conditions described in Fig 2. Data are presented as the percent compound remaining (y-axis). dCTP and ara-CTP are significantly hydrolyzed (p < 0.001). SMDU-TP and dGTP, in the same reaction tube, had no significant hydrolysis in the presence of SAMHD1. HPLC analysis of each nucleoside was done twice in triplicate. Mean and SEM are plotted with significant or no significant (n.s.) differences determined using T test analysis.