Abstract
The aim of this study was to evaluate the effect of vitamin D supplementation in women with hypovitaminosis D and “small burden” uterine fibroids.
This study focused on 208 women diagnosed with uterine fibroids and concomitant hypovitaminosis D, from January to December 2014. One hundred eight women of the initial study population were diagnosed with “small burden” uterine fibroids. Among them, those who underwent a proper vitamin D supplementation constituted the “study group” (n = 53), while women who spontaneously refused the therapy or did not perform it properly, constituted the “control group” (n = 55). The characteristics of uterine fibroids, the fibroid-related symptoms, and the vitamin D serum levels were evaluated 12 months after the initial diagnosis.
In women with uterine fibroids, a negative correlation emerged between the baseline 25-hydroxy-cholecalciferol (25-OH-D3) concentration and both the volume of the largest fibroid (r = −0.18, P = 0.01) and the total volume of fibroids (r = −0.19, P = 0.01). No correlation was found between the baseline 25-OH-D3 levels and the number of fibroids per patient (r = −0.10, P = 0.16). In women of the “study group,” a significant increase in the 25-OH-D3 serum level was observed after 12 months of supplementation, and a lower rate of surgical or medical treatment due to the “progression to extensive disease” was reported (13.2% vs 30.9%, P = 0.05).
Supplementation therapy with 25-OH-D3 restores normal vitamin D serum levels in women with “small burden” fibroids. In these women, vitamin D supplementation seems to reduce the progression to an extensive disease, and thus the need of conventional surgical or medical therapy.
Keywords: 25-OH-D3, fibroids, myomas, supplementation, vitamin D
1. Introduction
Uterine fibroids are the most common benign tumors of the female genital tract, and their prevalence during a woman's lifetime ranges from 50% to 80%.[1] Symptomatic women typically suffer from menstrual disorders, heavy menstrual bleeding, anemia, pelvic pain, and “bulky symptoms” (bladder or rectal pressure). Moreover, uterine fibroids seem to be related to infertility, early pregnancy loss, and several adverse obstetric outcomes.[2,3] The choice of the appropriate therapy for uterine fibroids is influenced by several factors, including the severity of symptoms, infertility, the tumor characteristics (number, size, and location), the patient's age, wish to preserve the uterus, and the desire of future pregnancies.[4]
Although uterine fibroids still represent the most common indication for hysterectomy and gynecological hospitalization, with an estimated annual cost of 34 billion dollars in the United States,[5] an effective conservative surgical approach[6] and the opportunity of a long-term medical treatment seem to be the most appropriate choices. Conventional medical treatments with gonadotropin-releasing hormone (GnRH) analogs are feasible only for short-term therapy, in order to avoid the potential risks and the menopausal-like disorders associated with prolonged therapy.[7] Recently, ulipristal acetate (UPA), a selective progesterone receptor modulator, has been introduced as a new medical strategy in the treatment of uterine fibroids with promising results.[4]
These medical treatments are commonly used for women with symptomatic uterine fibroids. However, even women with a limited (“small burden”) disease could benefit from a therapeutical approach in order to prevent the progression to an advanced and symptomatic disease. Obviously, such a therapeutical strategy in women with “small burden” uterine fibroids should not only be effective, but should also be low cost and low risk.[8] In this context, the potential role of vitamin D supplementation could be relevant.
Recent studies[6,7,9,10] suggest that hypovitaminosis D is associated with an increased risk of uterine fibroids. Moreover, vitamin D inhibits the growth and promotes the apoptosis of fibroid cells in “in vitro” studies, and it seems to reduce the fibroid size in “in vivo” animal models.[11–15] According to these findings, vitamin D supplementation might be an effective, safe, and low-cost therapy in the primary prevention and treatment of uterine fibroids, but its efficacy in humans needs to be carefully evaluated.
The aim of this study was to evaluate the opportunity of vitamin D supplementation in women with “small burden” uterine fibroids and hypovitaminosis D.
2. Methods
This study focused on women diagnosed with uterine fibroids at the pelvic ultrasound outpatient service of our institution, from January 2014 to December 2014. Women were referred to our ultrasound outpatient service in case of signs or symptoms suggestive of gynecological disorders (e.g., menstrual disorders, pelvic pain, infertility).
All the ultrasound examinations were performed with a high-resolution system (Voluson 730 GE Healthcare, Milwaukee, WI) with the use of a 3.5 to 5.5 mHz probe, by the same senior gynecologist.
Patients were initially eligible if they were diagnosed with at least 1 uterine fibroid with a mean diameter ≥10 mm, and only Caucasian women were considered. The characteristics of each patient's fibroids (number, size, and location) were accurately reported. In particular, 3 perpendicular diameters (d1, d2, and d3 in mm) were collected for each fibroid and, according to previous studies,[16,17] the volume of each fibroid (in cm3) was calculated with the ellipsoid formula: 4/3∗π∗(d1∗d2∗d3)/8. For each patient, the total volume of fibroids (defined as the sum of the volumes of all the fibroids detected) was also calculated.
The background and clinical characteristics of each patient were recorded: age, body mass index (BMI), tobacco use, and comorbidities were considered. Similarly, the obstetric and gynecological history and the lifestyle habits of each woman (with specific interest in dietary habits and sun exposure) were also recorded. Fibroids-related symptoms were accurately investigated, and, in particular, dysmenorrhea, pelvic pain, dyspareunia, and menstrual disorders were reported.
Among the women initially diagnosed with uterine fibroids, those with ongoing vitamin D supplementation for other clinical reasons (e.g., osteoporosis or previous diagnosis of hypovitaminosis D) were excluded. The remaining women, diagnosed with uterine fibroids, constituted the “initial study population.”
In these women, the 25-hydroxy-cholecalciferol (25-OH-D3), parathyroid hormone (PTH), and calcium serum levels were measured (the same day of the ultrasound examination). The 25-OH-D3 serum level was determined with a chemiluminescence enzyme-linked immunosorbent assay method (DRG Instruments GmbH, Marburg, Germany). The intra and interassay coefficients of variation were, respectively, 9.7% and 14.7%. The PTH serum level was determined with an electrochemiluminescent method (Elecsys 1010, coefficient of variation 4.0%) and the calcium level with a colorimetric assay (Hitachi Model 917 Multichannel Analyzer, coefficient of variation 1.75%) (Roche Diagnostics, Indianapolis, IN).
Hypovitaminosis D was defined as a 25-OH-D3 serum level < 30 ng/mL.[18] We properly recorded the season (autumn–winter or spring–summer) in which the 25-OH-D3, PTH, and calcium determination was obtained. All the women with hypovitaminosis D were offered standard supplementation therapy with 50,000 IU of cholecalciferol (oral solution) once per week for 8 weeks, followed by maintenance therapy of 2000 IU daily for a year.[19] Trained nurses conducted phone interviews at 3 and 6 months after the initial diagnosis to evaluate the adequacy of the therapy.
Among the “initial study population,” we focused on women diagnosed with “small burden” uterine fibroids. Therefore, we excluded women with uterine fibroids >50 mm in diameter, with more than 4 fibroids or with severe fibroids-related symptoms (which required a prompt surgical or medical treatment). Similarly, women with other concomitant gynecological disease (e.g., adnexal or endometrial pathology) or with unexplained infertility were excluded.
Twelve months after the initial diagnosis, in the same year period of the inclusion, all the women initially diagnosed with “small burden” uterine fibroids and hypovitaminosis D were called back for an ultrasound evaluation of the uterine fibroids. The women initially diagnosed with “small burden” uterine fibroids and hypovitaminosis D who properly performed the vitamin D supplementation therapy, constituted the “study group,” while women with “small burden” uterine fibroids and hypovitaminosis D who did not properly perform the therapy or refused it, constituted the “control group.”
The “progression to extensive disease” was defined by the increase in size of uterine fibroids (with the development of one or more fibroids >50 mm) or by a significant worsening of fibroids-related symptoms in the 12 months after the initial diagnosis. These women were offered a medical therapy with GnRH analogs or UPA, or a surgical procedure (hysterectomy or myomectomy). The women with a “progression to extensive disease” were excluded from the final analysis, as well as the women who reported a pregnancy in the 12 months after the initial diagnosis.
The composition of the “initial study population,” of the “study group” and the “control group,” is shown in Fig. 1.
Figure 1.
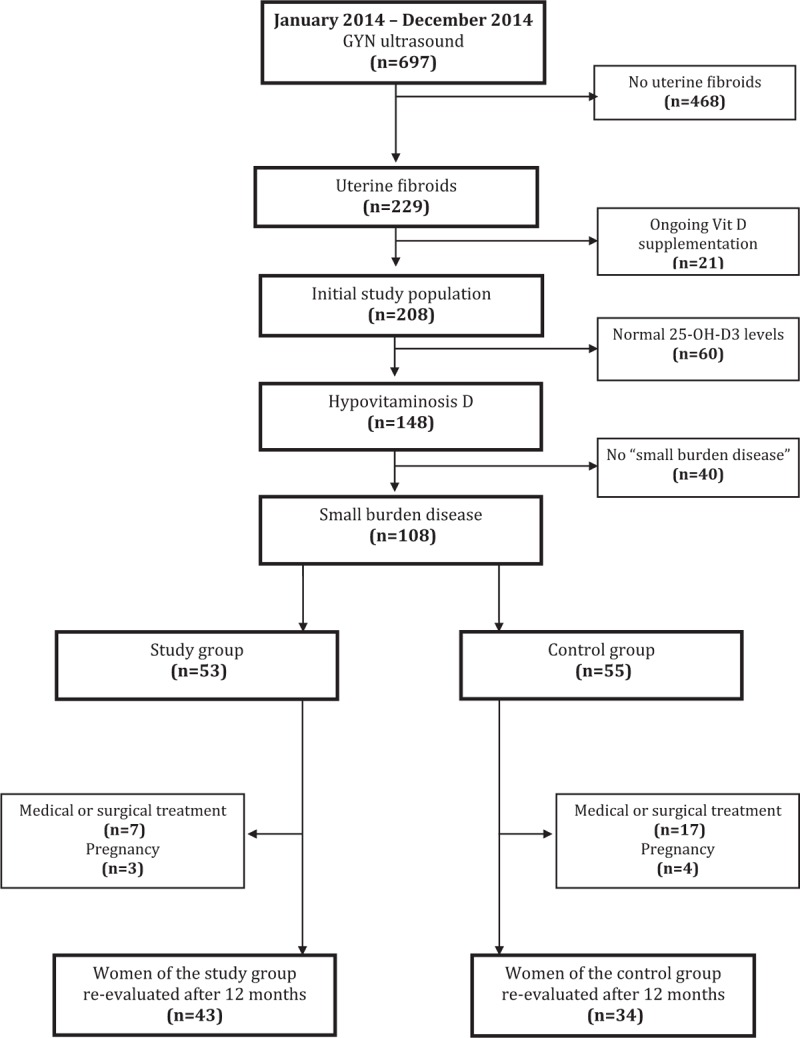
Composition of the study population, of the “small burden disease” subgroup, and of the “study group” and “control group” according to inclusion and exclusion criteria.
At the call back visit (12 months after the initial diagnosis), the 25-OH-D3, PTH, and calcium serum levels were measured both in the “study group” and in the “control group.” In particular, the changes in 25-OH-D3 serum levels (CL) in each patient were calculated as the difference between the final concentration (FC, measured 12 months after the initial diagnosis) and the starting concentration (SC, measured at the time of initial diagnosis). The increase (%) was defined by the formula: 100∗CL/SC.
As a secondary analysis, in relation to the achievement of normal 25-OH-D3 levels, we identified 2 subgroups of patients: “responders” and “nonresponders” (defined as those women who properly performed the vitamin D supplementation therapy for 12 months, and achieve or not normal 25-OH-D3 levels, respectively). These 2 subgroups were compared with respect to all the background, clinical and fibroids characteristics, in order to identify any factors associated with the attainment of normal levels of vitamin D after the supplementation therapy.
2.1. Statistical analysis
Statistical software SPSS 20 (SPSS Inc, Chicago, IL) was used for data analysis. All continuous variables were tested for normality with the D’Agostino-Pearson test. Normally distributed variables were expressed as mean ± standard deviation (SD), while skewed variables were reported as median and interquartile range (IQR). Mann–Whitney test, Wilcoxon test, or t test was used for comparison as appropriate. Qualitative variables were expressed as proportions and were compared with chi-squared or Fisher exact test as appropriate. Pearson coefficient was determined for correlation between continuous variables and a linear regression was used to adjust for confounders when appropriate. A P < 0.05 was considered statistically significant.
2.2. Informed consent
All the women gave their signed informed consent for ultrasound execution; 25-OH-D3, PTH, and calcium serum levels determination; vitamin D supplementation; and data collection at the time of enrollment (when the initial diagnosis of uterine fibroids was made). The local ethic committee approval was obtained.
3. Results
During the study period, 697 women underwent a gynecological ultrasound scan at our institution, and 229 of them (32.6%) were diagnosed with uterine fibroids. At the time of initial diagnosis, 21 patients had an ongoing vitamin D supplementation for other clinical reasons (e.g., osteoporosis) and were excluded. The remaining 208 women, without previous diagnosis of hypovitaminosis D, constituted the “initial study population.”
In these women, the baseline 25-OH-D3, PTH, and calcium serum levels were measured. The mean (±SD) 25-OH-D3 serum level was 25.7 (±10.7) ng/mL, and 148 patients (71.1%) were diagnosed with hypovitaminosis D. No difference in the season of the determination emerged (48.1% autumn–winter vs 51.9% spring–summer, P = 0.42). A significant difference in the mean 25-OH-D3 serum level between the women with hypovitaminosis D (n = 148) and the women with normal vitamin D serum levels (n = 60) was reported (20.4 ± 5.9 vs 39.0 ± 8.0 ng/mL; P < 0.001). To all of the women diagnosed with hypovitaminosis D, a standard vitamin D supplementation therapy[19] was offered, routinely.
In the women of “initial study population,” the mean (±SD) PTH serum level was 47.9 (±15.9) ng/L and the mean (±SD) calcium serum level was 8.9 (±0.8) mg/dL.
3.1. Initial study population
Among the initial study population (n = 208), a total of 370 uterine fibroids were identified; in particular, 125 patients (60.1%) presented a single fibroid, while multiple fibroids were detected in 83 patients (39.9%). Moreover, a total of 259 fibroids were detected in women with hypovitaminosis D while 111 fibroids were detected in women with normal vitamin D serum levels.
A negative correlation emerged between the baseline 25-OH-D3 concentration and both the volume of the largest fibroid (r = −0.18, P = 0.01) and the total volume of fibroids (r = −0.19, P = 0.01). No correlation was found between 25-OH-D3 baseline levels and the number of fibroids per patient (r = −0.10, P = 0.16). Furthermore, no correlation was found between PTH serum levels and the volume of the largest fibroid, the total volume of fibroids, and the number of fibroids per patient (r = −0.02, P = 0.73; r = −0.02, P = 0.76; and r = −0.03, P = 0.66, respectively). Similarly, no correlation emerged between calcium serum levels and the volume of the largest fibroid, the total volume of fibroids, and the number of fibroids per patient (r = −0.03, P = 0.71; r = −0.03, P = 0.68; and r = −0.03, P = 0.68, respectively).
3.2. Women with “small burden” uterine fibroids
Among the women of the “initial study population” with hypovitaminosis D (n = 148), 108 patients constituted the “small burden” disease subgroup. As previously described, all of these women were offered a standard vitamin D supplementation therapy because of hypovitaminosis D.[19]
Among women with “small burden” disease and hypovitaminosis D, 53 patients performed the therapy adequately and constituted the “study group” (Fig. 1). In the 12 months after the initial diagnosis, 3 women of the study group got pregnant (5.7%) and 7 women (13.2%) underwent traditional medical or surgical therapy because of the “progression to extensive disease” (2 women underwent surgical procedures and 5 underwent medical therapy).
The remaining 55 women (16 who refused the supplementation therapy and 39 who did not perform it adequately) constituted the “control group.” In the 12 months after the initial diagnosis, 4 women of the “control group” got pregnant (7.3%) and 17 women (30.9%) underwent medical or surgical therapy because of the “progression to extensive disease” (6 women underwent surgical procedures and 11 underwent medical therapy).
In the “study group,” no difference in the baseline vitamin D levels emerged between patients with or without “progression to extensive disease” (21.1 ± 5.1 vs 19.9 ± 6.9, P = 0.44). Even in the “control group,” no difference in the baseline vitamin D levels emerged between patients with or without “progression to extensive disease” (20.6 ± 5.5 vs 21.4 ± 8.4, P = 0.71).
The rate of women with “progression to extensive disease” was significantly lower in the “study group” compared with controls (13.2% vs 30.9%, P = 0.05). The rate of women who got pregnant in the 12 months after the initial diagnosis was similar (5.7% vs 7.3%, P = 0.96).
The remaining 43 women in the “study group” and 34 in the “control group” underwent an ultrasound evaluation and a 25-OH-D3, PTH, and calcium serum levels determination (12 months after the initial diagnosis). None of these women reported changes in diet and sun exposure. No sign of vitamin D toxicity was reported in any patient.
3.3. Vitamin D serum levels after supplementation therapy
Considering the 43 women of the “study group” for which the call back visit was performed, the mean (±SD) baseline 25-OH-D3 serum level was 19.9 (±1.0) ng/mL, the mean (±SD) baseline PTH serum level was 51.5 (±9.2) ng/L, and the mean (±SD) baseline calcium serum level was 8.7 (±1.0) mg/dL. At the call back visit (performed 12 months after the starting of appropriate vitamin D supplementation therapy), the mean 25-OH-D3 serum level in these women was 30.7 ± 10.5 ng/mL; thus the difference was statistically significant (19.9 ± 1.0 vs 30.7 ± 10.5 ng/mL; P < 0.001). In the women of the “study group,” the median (IQR) CL was 9.2 (3.5–18.7) ng/mL, with a median (IQR) increase of 46% (15.6–100). A significant negative correlation emerged between 25-OH-D3 baseline levels and the increase (%) with an r coefficient of −0.72 (P < 0.001) (Fig. 2). In these 43 women of the “study group,” the mean (±SD) PTH and calcium serum levels measured 12 months after the initial diagnosis were not significantly different from baseline levels (51.5 ± 9.2 vs 51.0 ± 15.9, P = 0.23 and 8.7 ± 1.0 vs 8.6 ± 0.9, P = 0.55, respectively).
Figure 2.
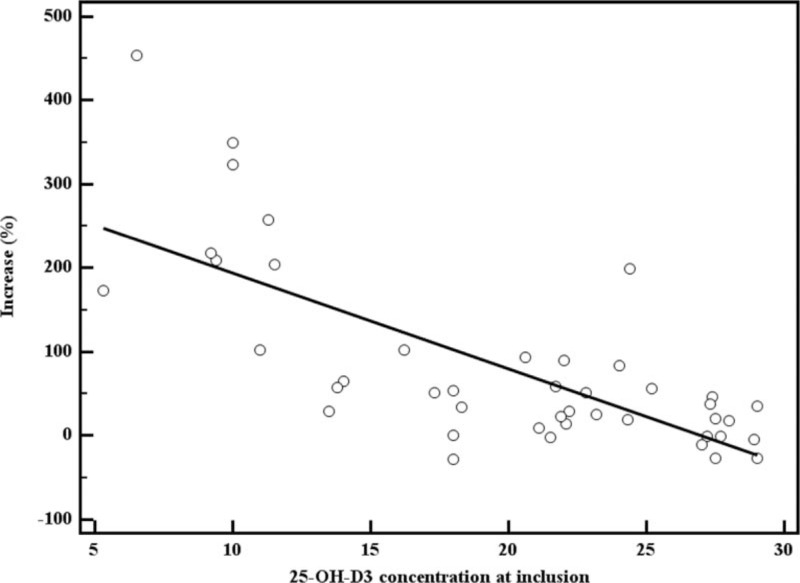
Correlation between 25-OH-D3 baseline levels and increase (%) in the “study group” (n = 43). 25-OH-D3 = 25-hydroxy-cholecalciferol.
In the 34 women of the “control group” for which the call back visit was performed, the mean (±SD) baseline 25-OH-D3 serum level was 21.4 ± 8.4 ng/mL. At the call back visit (performed 12 months after the initial diagnosis), the mean 25-OH-D3 serum level was 23.1 ± 7.6 ng/mL; thus, no significant difference was reported (21.4 ± 8.4 vs 23.1 ± 7.6 ng/mL; P = 0.38). In these 34 women, the mean (±SD) PTH and calcium serum levels measured 12 months after the initial diagnosis were not significantly different from baseline levels (47.5 ± 11.9 vs 47.2 ± 8.8, P = 0.08 and 8.6 ± 0.8 vs 8.5 ± 1.2, P = 0.15, respectively).
3.4. Characteristics of uterine fibroids after supplementation therapy
Considering the 43 women of the “study group” and the 34 women of the “control group” for which the call back visit was performed, a total of 71 fibroids were identified in the women of the “study group” and 68 in the women of the “control group.” The number, topographic site, and location of all lesions remained the same between the initial and the final ultrasounds. Table 1 reports the median (IQR) diameter and volume at inclusion and after 1 year of therapy both in the “study group” and in the “control group.” A significant increase in fibroids diameter and volume was found in women of the “control group” (P < 0.001), while no significant difference in diameter or volume of fibroids emerged in women of the “study group.” Figure 3 reports the median (IQR) fibroids diameter both in the “study group” and in the “control group,” at baseline and after 1 year.
Table 1.
Vitamin D, PTH, calcium serum levels, and fibroids characteristics in the study group (43 women, with a total of 71 fibroids) and the control group (34 women, with a total of 68 fibroids) at inclusion and after 1 year.
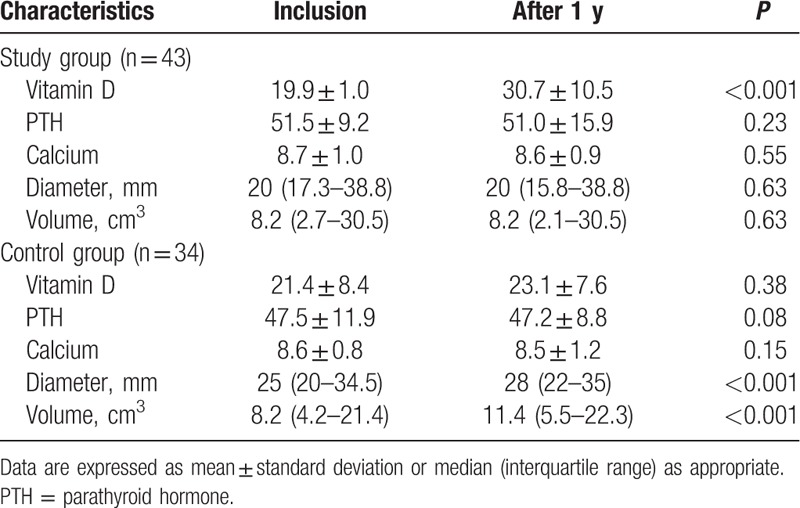
Figure 3.
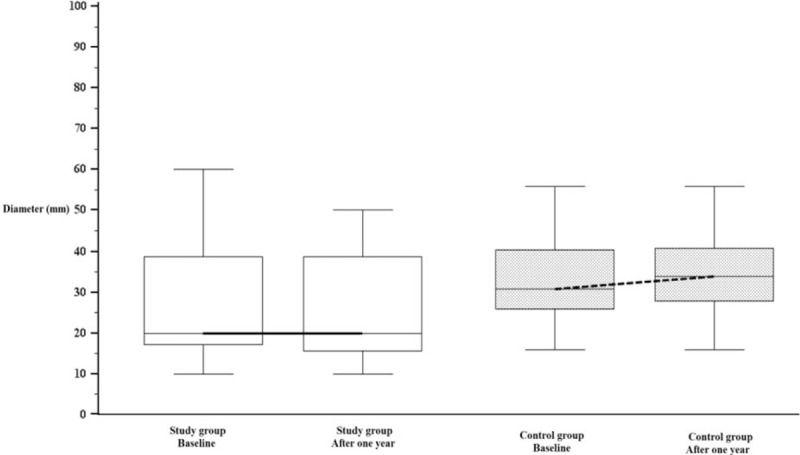
Fibroids median (interquartile range) diameter in the “study group” and in the “control group”, at baseline and after 1 year.
In the 43 patients of the study group for which the call back visit was performed, no correlation between 25-OH-D3 serum level at 1 year and the volume of largest fibroid (r = −0.07, P = 0.68), the total fibroid volume (r = −0.07, P = 0.6619), or the number of fibroids per patient (r = 0.004, P = 0.9799) was noted, even adjusting for responders/nonresponders (linear regression).
On the contrary, in the 34 women of the “control group” for which the call back visit was performed, we found a significant correlation between 25-OH-D3 levels at 1 year, the volume of largest fibroid and the total fibroid volume (r = −0.42, P = 0.02 and r = −0.45, P = 0.01, respectively). No correlation emerged with the number of fibroid per patient (r = −0.05, P = 0.80).
3.5. Secondary analysis
One year after the initial diagnosis, considering the 43 women of the “study group” for which the call back visit was performed, 18 patients (41.9%) reached normal levels of 25-OH-D3 (“responders”), while 25 (58.1%) did not reach a normal level (“nonresponders”).
The “responders” had a baseline 25-OH-D3 serum level of 20.3 ± 7.4 ng/mL and then 40.1 ± 9.1 ng/mL after 1 year of therapy (P < 0.001). The “nonresponders” had a starting 25-OH-D3 level of 19.6 ± 6.7 ng/mL and a final level of 23.9 ± 4.7 ng/mL (P = 0.01). No significant differences in background or clinical characteristics between the “responders” and “nonresponders” emerged, with the exception of the prevalence of cystic mastopathy (Table 2). The mean (±SD) baseline PTH serum level was similar between “responders” and “nonresponders” (47.9 ± 15.6 vs 54.1 ± 21.4; P = 0.3), as well as the mean (±SD) PTH serum level 12 months after appropriate vitamin D supplementation (46.2 ± 12.9 vs 54.4 ± 17.2; P = 0.1). Similarly, even the calcium serum levels at the baseline and at 12 months were similar between “responders” and “nonresponders” (8.8 ± 0.5 vs 8.6 ± 1.2; P = 0.5 and 8.7 ± 1.0 vs 8.6 ± 0.8; P = 0.7, respectively).
Table 2.
Background and clinical characteristics in “responders” and “nonresponders”.
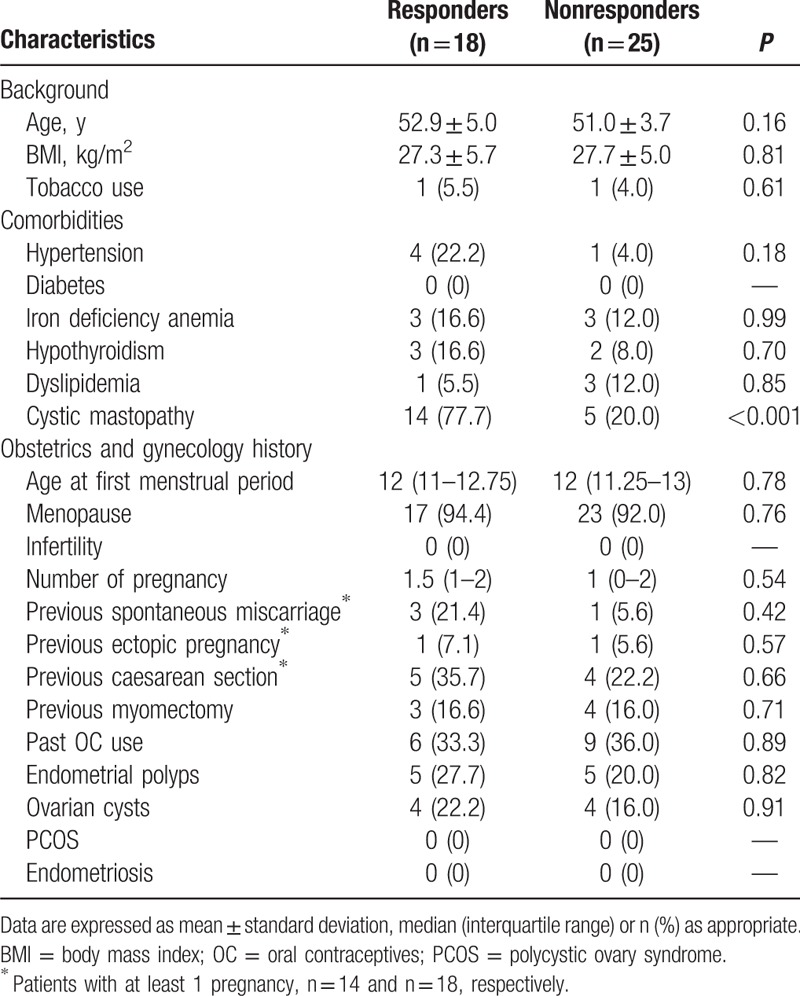
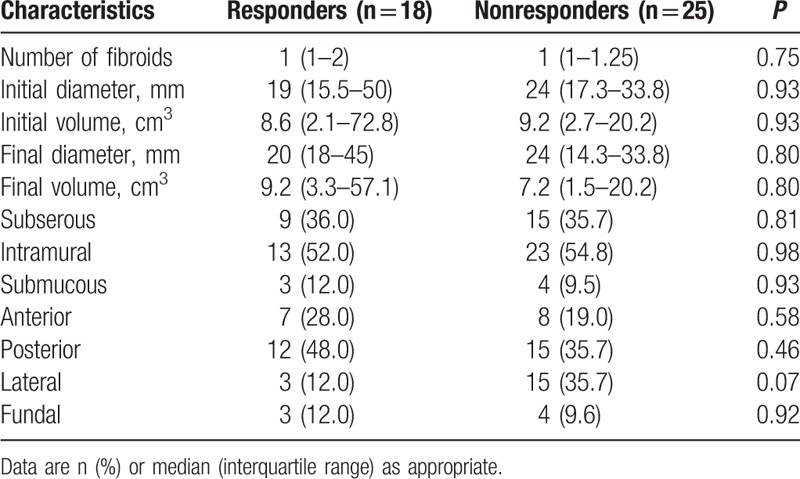
Among the “responders,” 4 patients (22.2%) presented a single fibroid, while multiple fibroids were detected in 14 patients (77.8%). Among the “nonresponders,” 7 women (28%) presented a single fibroid, while multiple fibroids were detected in 18 patients (72%). Hence, a total of 25 fibroids were detected in the “responders,” while 42 fibroids were detected in the “nonresponders.” The characteristics of uterine fibroids in the 2 groups were similar (Table 3).
Table 3.
Fibroids characteristics in “responders” and “nonresponders”.
4. Discussion
Hypovitaminosis D has recently been associated with a higher prevalence of uterine fibroids, and some authors reported that vitamin D serum levels are inversely correlated with the severity of uterine fibroids.[20] Besides vitamin D, even PTH serum levels could be related to uterine fibroids. Previous studies reported a higher expression of PTHrP (a protein with N-terminal homology to PTH) in fibroids than in normal myometrium.[21] Both PTHrP and PTH act via a receptor with 7-transmembrane spanning domains that is capable of stimulating both adenylate cyclase and phospholipase C.[22] Hence, PTHrP, as well as PTH, may play a role in regulating fibroid growth or differentiation. However, to our knowledge, no previous “in vivo” study confirmed a correlation between PTH serum levels and uterine disease. Our results confirmed these data. Indeed, no correlation was found between PTH serum levels and the characteristics of uterine disease (volume of largest fibroid, total volume of fibroids, and the number of fibroids per patient).
On the other hand, our results confirm the correlation between hypovitaminosis D and uterine fibroids since we found that lower levels of vitamin D are related to a more severe uterine disease, in terms of volume of the largest fibroid (r = −0.18, P = 0.01) and total volume of fibroids (r = −0.19, P = 0.01).
Recent “in vitro” studies have explored the potential role of vitamin D in the development of uterine fibroids. It has been demonstrated that vitamin D inhibits the growth of fibroid cells through the down-regulation of proliferating cell nuclear antigen (PCNA), cyclin-dependent kinase 1, and B-cell lymphoma 2 (and suppresses the catechol-O-methyltransferase expression and its activity).[11] In addition, vitamin D seems to reduce the transforming growth factor beta-3 effects on the process of fibrosis in human fibroid cells[12] and to limit the aberrant expression of major extracellular matrix-associated proteins.[14] Moreover, in 2012, Halder et al found that treatment with 1,25-OH-D3 significantly reduces fibroid tumor size in Eker rats by suppressing cell growth and proliferation-related genes, antiapoptotic genes, and estrogen and progesterone receptors.[13] These data are of particular clinical relevance, since vitamin D could be a potential safe, nonsurgical therapy for the treatment of uterine fibroids.[7] However, to date, no data are available on the effectiveness and safety of vitamin D supplementation in women affected by uterine fibroids.
First of all, our preliminary data suggest that 25-OH-D3 is effective in restoring normal levels of vitamin D in women with uterine fibroids. After 1 year of therapy, the mean level of 25-OH-D3 in the study group was significantly higher compared with the baseline level (30.7 ± 10.5 vs 19.9 ± 7.0 ng/mL, P < 0.001). Moreover, the efficacy of supplementation seems to be related to the baseline vitamin D serum levels, with higher responses in women with lower levels at the time of initial diagnosis (Fig. 2); this datum appears to be consistent with previous studies.[23] Therefore, according to our results, the presence of uterine fibroids does not seem to affect the response to vitamin D supplementation.
In our cohort, among the women who underwent an appropriate supplementation therapy, 58.1% of them did not reach normal levels of 25-OH-D3. The effectiveness of an appropriate repletion strategy to reach the normal 25-OH-D3 concentration is highly variable, and ranged from 5% to 89% in previous studies.[24] The reasons for this high variability are currently unknown. It is reported in the literature that the serum 25-OH-D3 response to vitamin D supplementation could be related to genetic factors, BMI, and baseline levels,[23] but the potential impact of uterine fibroids on the effectiveness of vitamin D supplementation is currently unknown.
In our cohort, we did not find any significant differences in background and clinical characteristics between “responders” and “nonresponders.” Moreover, the failure to achieve normal levels of vitamin D seems not to be related to the characteristics of uterine fibroids. Hence, additional genetic factors could be involved and should be properly investigated in future studies, in order to identify patients with lower response to 25-OH-D3 supplementation.
The main aim of this study was to evaluate the effect of vitamin D supplementation (administered because of the diagnosis of hypovitaminosis D) in women with “small burden” uterine fibroids.
Analyzing the potential effects of vitamin D supplementation on uterine fibroids, we noted that among the women with “small burden” disease and hypovitaminosis D who performed an adequate vitamin D supplementation (“study group”), a lower rate of “progression to extensive disease,” with the need of surgical or medical treatment, was observed in the 12 months after the initial diagnosis. This could mean that women with uterine fibroids who underwent vitamin D supplementation had a lower risk of progression of uterine disease.
The effect of vitamin D supplementation was also examined by comparing the sonographic features of uterine fibroids at inclusion and 12 months after the initial diagnosis, both in patients who had properly performed the therapy (“study group”) and in those who did not adequately perform the therapy (“control group”). No significant differences were found in diameter or in volume of the identified fibroids in the study group, while a slight but significant increase in diameter and volume was noted in the control group. Thus, the growth pattern of fibroids with “small uterine burden” under supplementation with 25-OH-D3 seems to be stable, with no increases or decreases in size or number of identified lesions. Instead, women with “small burden” uterine fibroids who did not perform appropriate vitamin D supplementation seem to have a slight but significant increase in size of the lesions and a higher need for subsequent medical or surgical therapy.
In our cohort, we found a relatively high prevalence of perimenopausal and menopausal women, conditions that may make less noticeable the effect of supplementation on fibroids. However, the common assumption that uterine fibroids (and their related symptoms) will be resolved by the approach of menopause may not always be valid.[25] A recent review by Ciarmela et al[26] reported that women in the perimenopausal period should be considered to have a higher risk of developing fibroids, and that for these women it is also plausible that existing fibroids may continue to grow. Moreover, immediately before menopause, women can experience several months or years of estrogen-dominated menstrual cycles, with a risk of a growth spurt of fibroids, considering their estrogen sensibility.[26]
Given this datum, the plausible stabilizing effect of vitamin D supplementation on size and number of “small burden” uterine fibroids could be most beneficial, especially in pre and perimenopausal women. The aim would be to prevent the possible growth of fibroids and the onset of fibroid-related symptoms, as well as to reduce the need for surgery or conventional medical therapy.
Further possible beneficial effects of vitamin D supplementation in women with hypovitaminosis D and uterine fibroids might become evident with longer duration of therapy or with different dosages of 25-OH-D3. However, additional studies are needed.
This study has some potential limitations and it should be considered as a “preliminary” study. We considered women with uterine fibroids and incidental diagnosis of hypovitaminosis D (for whom a supplementation therapy with 25-OH-D3 was routinely recommended), and we evaluate the effect of such a therapy on fibroids. Thus the analysis of the potential effect of vitamin D supplementation on uterine fibroids is based on whether patients followed or did not follow the treatment. This “as treated analysis” could lead to potential bias, since the patient's compliance can be different because the characteristics of compliers and noncompliers can be different.
Obviously, in order to investigate the potential role of vitamin D supplementation therapy in women with uterine fibroids, appropriate well-designed randomized controlled trials (RCTs) should be performed. However, our results could represent a starting point for future bigger and well-designed trials.
The small sample size is another potential limitation of this study as well as the potential inaccuracy of the sonographic method of evaluation and measurement of fibroids, which inevitably has an ultrasound-related degree of imprecision. However, all ultrasound examinations were performed by the same senior sonographer, and we used a standard technique for identification and measurement of fibroids.
The initial assessment of vitamin D serum levels was not performed in the same year period for each woman; however, no difference in the season of determination emerged. Moreover, in order to eliminate potential seasonal confounding effects, we repeated the vitamin D determination after 1 year, in the same year period of inclusion.
To our knowledge, this is the first evaluation of an “in vivo” effect of vitamin D supplementation in women with uterine fibroids, which confirms the hypothesis that it could be a safe, low cost, and low-risk treatment for fibroids stabilization, preventing the progression to more severe and symptomatic conditions. However, appropriate and well-designed RCTs are desirable, in order to confirm the effectiveness of vitamin D supplementation in inhibiting or stabilizing the growth of uterine fibroids, and to better clarify the optimal dosage and duration of such a therapy.
Acknowledgments
The authors wish to thank the Department of Biomedical Sciences and Public Health, Section of Hygiene, Preventive Medicine and Public Health, Polytechnic University of Marche, Ancona, Italy for the statistical consultation during the drafting of the present work.
Footnotes
Abbreviations: 25-OH-D3 = 25-hydroxy-cholecalciferol, BMI = body mass index, CL = changes in 25-OH-D3 serum levels, FC = final 25-OH-D3 concentration, GnRH = gonadotropin-releasing hormone, IQR = interquartile range, IU = international unit, PCNA = proliferating cell nuclear antigen, PCOS = polycystic ovary syndrome, PTH = parathyroid hormone, RCTs = randomized controlled trials, SC = starting 25-OH-D3 concentration, UPA = ulipristal acetate.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–7. [DOI] [PubMed] [Google Scholar]
- [2].Carranza-Mamane B, Havelock J, Hemmings R, et al. The management of uterine fibroids in women with otherwise unexplained infertility. J Obstet Gynaecol Can 2015;37:277–88. [DOI] [PubMed] [Google Scholar]
- [3].Ciavattini A, Clemente N, Delli Carpini G, et al. Number and size of uterine fibroids and obstetric outcomes. J Matern Fetal Neonatal Med 2015;28:484–8. [DOI] [PubMed] [Google Scholar]
- [4].Trefoux Bourdet A, Luton D, Koskas M. Clinical utility of ulipristal acetate for the treatment of uterine fibroids: current evidence. Int J Womens Health 2015;7:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cardozo ER, Clark AD, Banks NK, et al. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012;206:e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Al-Hendy A, Badr M. Can vitamin D reduce the risk of uterine fibroids? Womens Health (Lond) 2014;10:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brakta S, Diamond JS, Al-Hendy A, et al. Role of vitamin D in uterine fibroid biology. Fertil Steril 2015;104:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wu JL, Segars JH. Is vitamin D the answer for prevention of uterine fibroids? Fertil Steril 2015;104:559–60. [DOI] [PubMed] [Google Scholar]
- [9].Paffoni A, Somigliana E, Vigano’ P, et al. Vitamin D status in women with uterine leiomyomas. J Clin Endocrinol Metab 2013;98:E1374–8. [DOI] [PubMed] [Google Scholar]
- [10].Baird DD, Hill MC, Schectman JM, et al. Vitamin D and the risk of uterine fibroids. Epidemiology 2013;24:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sharan C, Halder SK, Thota C, et al. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril 2011;95:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab 2011;96:E754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Halder SK, Sharan C, Al-Hendy A. 1,25-Dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod 2012;86:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Halder SK, Osteen KG, Al-Hendy A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum Reprod 2013;28:2407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Halder SK, Osteen KG, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces extracellular matrix associated protein expression in human uterine fibroid cells. Biol Reprod 2013;89:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].De Vivo A, Mancuso A, Giacobbe A, et al. Uterine myomas during pregnancy: a longitudinal sonographic study. Ultrasound Obstet Gynecol 2011;37:361–5. [DOI] [PubMed] [Google Scholar]
- [17].Benaglia L, Cardellicchio L, Filippi F, et al. The rapid growth of fibroids during early pregnancy. PLoS ONE 2014;9:e85933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Matyjaszek-Matuszek B, Lenart-Lipińska M, Woźniakowska E. Clinical implications of vitamin D deficiency. Prz Menopauzalny 2015;14:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- [20].Sabry M, Halder SK, Allah AS, et al. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health 2013;5:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Weir EC, Goad DL, Daifotis AG, et al. Relative overexpression of the parathyroid hormone-related protein gene in human leiomyomas. J Clin Endocrinol Metab 1994;78:784–9. [DOI] [PubMed] [Google Scholar]
- [22].Abou-Samra AB, Jüppner H, Force T, et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA 1992;89:2732–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Didriksen A, Grimnes G, Hutchinson MS, et al. The serum 25-hydroxyvitamin D response to vitamin D supplementation is related to genetic factors, BMI, and baseline levels. Eur J Endocrinol 2013;169:559–67. [DOI] [PubMed] [Google Scholar]
- [24].Traub ML, Finnell JS, Bhandiwad A, et al. Impact of vitamin D3 dietary supplement matrix on clinical response. J Clin Endocrinol Metab 2014;99:2720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Giannubilo SR, Ciavattini A, Petraglia F, et al. Management of fibroids in perimenopausal women. Curr Opin Obstet Gynecol 2015;27:416–21. [DOI] [PubMed] [Google Scholar]
- [26].Ciarmela P, Ciavattini A, Giannubilo SR, et al. Management of leiomyomas in perimenopausal women. Maturitas 2014;78:168–73. [DOI] [PubMed] [Google Scholar]


