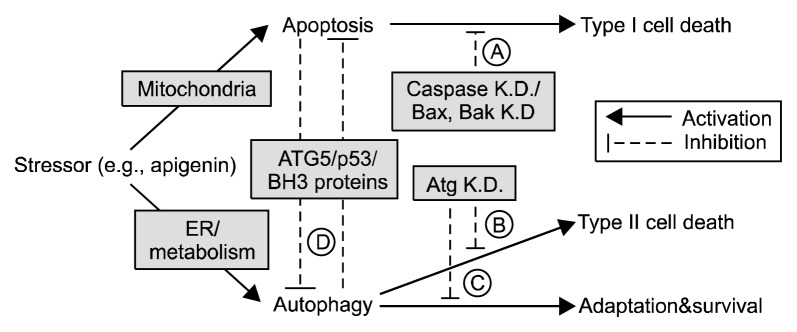
Figure 2.
Signaling pathways and molecules involved in crosstalk between apoptosis and autophagy. Cellular stressors such as apigenin can start mitochondria outer membrane permeabilization and subsequent cytochrome c release and apoptosis induction, while nutrient deficiency or ER stress can cause autophagy activation. Under physiological conditions, apoptosis and autophagy keep each other inactive through mutual inhibition. A strong apoptotic stimulus (for example DNA damage, death-receptor stimulation, or cytokine deprivation) can drive a cell into apoptotic ‘type I’ cell death. If apoptosis is inhibited under such conditions (by caspase knockout or Bax/Bak knockout, [A]), autophagy can become activated and result in a delayed ‘type II’ cell death through degradation of most cytoplasmic cell components and organelles. Under these circumstances, the K.D. of autophagy related genes [B] reduces cell death. Autophagy can become activated through ER stress (for example, accumulation of misfolded proteins in the ER or intracellular calcium release from the ER) or nutrient deficiency. The cell then ensures survival by enhancing metabolic recycling through autophagy and adapting to the new nutrient conditions. K.D. of autophagy genes in such a situation leads to an increase in apoptotic ‘type I’ cell death [C]. The crosstalk between apoptosis and autophagy [D] is mediated via proteolytic processing of ATG5, the transcription factor p53, and the binding and subcellular localization of Bcl-2 family proteins with BH3 domains. Data from Jaeger and Wyss-Coray.92 ER, endoplasmic reticulum; K.D., knockdown.