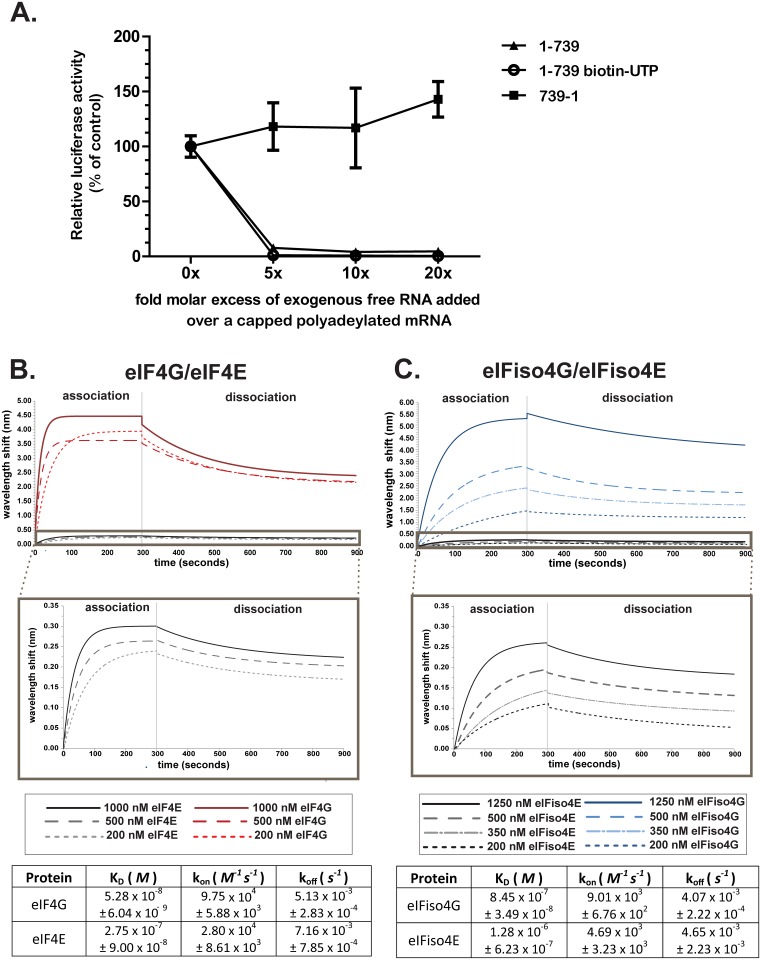
Fig 1. The TriMV 5’UTR RNA binds to eIF4G and eIFiso4G with greater affinity than the cap binding proteins.
Bio-layer interferometry (BLI) was used to measure the binding kinetics between purified recombinant wheat eIF4G, eIFiso4G, eIF4E, or eIFiso4E protein and the viral 5’ UTR sequence (nt 1–739). Biotin-labeled RNA was bound to a streptavidin biosensor and applied to solutions containing different concentrations of proteins. A) Relative luciferase activity in wheat germ of a m7GpppG-capped and polyadenylated vector reporter in the presence of competing free RNAs in increasing molar excess A 0- to 20-fold molar excess of the competing free RNAs corresponding to the biotin-UTP labeled and unlabeled TriMV 5’UTR sequence (1–739) and the non-functional TriMV reverse sequence (739–1) were used. In B and C are shown the BLI sensograms revealing the binding curves for eIF4G/eIF4E (B) and eIFiso4G/eIFiso4E (C). A magnification of the binding curves for eIF4E and eIFiso4E is included. A table with their corresponding kinetics values is displayed below the appropriate graphs.