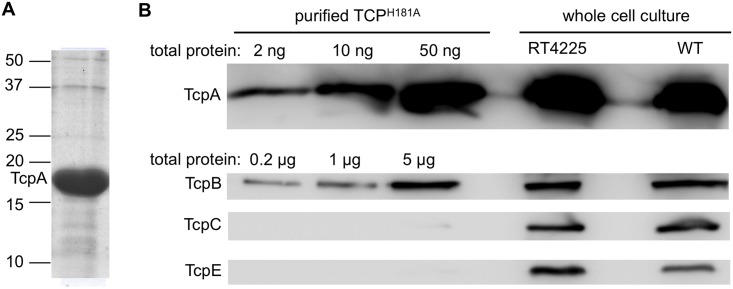
Fig 4. Co-localization of TcpB with purified TCPH181A.
(A) Coomassie-stained SDS gel of TCPH181A purified from V. cholerae RT4225, which has a mutation encoding a His181Ala substitution in the major pilin, TcpA. The protein concentration of the solution was found by spectrophotometry (A280) to be 10 mg/ml. The lane was overloaded with 90 μg of protein to show the purity, estimated at >95%. The positions of mass markers are indicated. The protein band at ~50 kDa is not likely to be TcpB (46 kDa), which based on the TcpA:TcpB stoichiometry determination (see below) is present in quantities too small to detect by Coomassie stain. (B) Immunoblots of pilins and pilus assembly components in purified TCPH181A and whole cell culture. For the purified TCPH181A samples, the total amount of protein loaded is indicated; for the whole cell culture samples, 25 μl was loaded into each well. Blots were probed with antibodies against TcpA and TcpB; TcpC and TcpE were also probed to assess contamination from outer and inner membranes, respectively. All blots were developed for 2 minutes.