Summary
Background
The Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) clinical trial documented that metformin plus rosiglitazone, but not metformin plus lifestyle intervention, provided superior durability of glycemic control relative to metformin monotherapy.
Objectives
We examined weight changes among TODAY participants that completed at least 6 months of treatment, evaluated predictors of lifestyle outcome, and examined whether weight changes were related to cardiometabolic outcomes across treatment arms.
Methods
The 595 youth with type 2 diabetes, (85.1% of randomized participants aged 11–17 years) completed assessments of weight-related and cardiometabolic measures at months 0, 6, 12 and 24. Repeated measures models were used to investigate associations over time.
Results
Lifestyle intervention did not enhance outcome relative to metformin alone and no predictors of response to lifestyle treatment were identified. However, changes in percent overweight across treatment arms were associated with changes in multiple cardiometabolic risk factors, and decreases of ≥7% in overweight were associated with significant benefits over 24 months.
Conclusions
Although adjunctive intensive lifestyle intervention did not improve weight-related outcomes, weight changes in the full TODAY sample were associated with small, but significant improvements in cardiometabolic status, highlighting the importance of optimizing weight management in youth with T2DM.
Keywords: type 2 diabetes, lifestyle, cardiometabolic risk, behavioral weight control
Introduction
Increases in the prevalence of type 2 diabetes mellitus (T2DM) in children and adolescents have accompanied increases in the prevalence of obesity. Given concerns about diabetes complications and the consequences for the adult health of affected youth, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) funded a randomized clinical trial, Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY). The goal of TODAY was to evaluate the efficacy of three treatment arms [metformin (M) monotherapy, metformin plus rosiglitazone (M+R), and metformin plus an intensive lifestyle program (M+L) on time to treatment failure [defined as hemoglobin A1c (HbA1c) ≥8% over 6 months or inability to wean from temporary insulin therapy within 3 months following acute metabolic decompensation] in recently diagnosed youth with T2DM.
Results demonstrated that adding rosiglitazone to metformin, but not an adjunctive lifestyle program, was associated with more durable glycemic control among youth who were followed for an average of 3.86 years (1). Although TODAY intensive lifestyle participants lost significantly more weight than those randomized to the other treatment arms by month 6, the point at which lifestyle programs typically achieve maximal weight changes, the benefits of lifestyle intervention for weight changes were not sustained at months 12 or 24 (1).
A substantial number of TODAY participants were unable to maintain adequate glycemic control during the first 6 months of the trial; these participants were characterized by lower β-cell reserve at randomization and did not benefit from any of the TODAY randomized treatments and were discontinued (2). Consequently, participants characterized by early non-response did not have exposure to intensive weekly sessions at the start of the lifestyle intervention. We examined outcomes of participants who had at least 6 months of exposure to their randomized treatment, and identified factors associated with more successful weight-related outcomes in the lifestyle treatment arm. To address the important question of whether weight loss should be a goal in the management of pediatric T2DM, we examined whether changes in weight-related measures were associated with changes in cardiometabolic risk factors across treatment arms.
Methods
Details regarding the TODAY design and methods have been reported (3). In brief, 699 youth aged 10–17 years were enrolled between July 2004 and February 2009. Participants had T2DM of less than 2 years duration (average 7.8 months) using criteria of the American Diabetes Association, a body mass index (BMI) ≥85th percentile for age and sex, (4) and were negative for diabetes autoantibodies. Participants were weaned from non-study medication and metformin initiated (at least 500 mg twice daily to the study dose of 1000 mg twice daily) during a run-in of 2–6 months. During run-in, participants had to demonstrate mastery of standard diabetes education, adhere to study procedures and attain glycemic control defined as HbA1c <8% for two consecutive months.
The TODAY protocol was approved by the Institutional Review Board at each participating site. Parents provided written informed consent; children and adolescents provided assent for study procedures.
Measures
Research assessments were obtained at clinic visits at months 0, 6, 12 and 24.
Anthropometric outcomes
Height was measured without shoes using a stadiometer. Weight was measured twice using a Seca scale (model 882, Seca USA, Hanover, MA), with a third measurement taken if the first two differed by >.2kg, and measurements were averaged. BMI was calculated [weight (kg) / [height (m)2], and percent overweight defined as BMI minus BMI at the 50th percentile for age and sex, divided by BMI at the 50th percentile times 100. Percent overweight was utilized as the primary weight-related metric because the expression of BMI values as a percentage of the 95th percentile has been recommended, and now is widely used, for describing and tracking heavier children (5).
Cardiometabolic outcomes
Assays were performed at the Northwest Lipid Research Laboratory as previously described (3). HbA1c, fasting lipids and 2-hour oral glucose tolerance tests (OGTTs) were obtained after a 10–14 h overnight fast. OGTTs were not obtained at month 12 per study protocol. The oral disposition index (oDI), a measure of β-cell function relative to insulin sensitivity was calculated as the product of insulin sensitivity (defined as 1/fasting insulin; 1/IF), multiplied by the c-peptide index (defined as the ratio of the incremental c-peptide and glucose responses over the first 30 min of the OGTT test; ΔC30/ΔG30) (2). Blood pressure was recorded three times (using a CAS 740 monitor with standardized oscillometric cuff sizes), and the average of the second and third recordings was used for analysis.
Lifestyle program
The lifestyle program has been described in detail (6). TODAY staff members delivered the program to youth participants and a family support person, most of whom were parents. Participants attended weekly intervention sessions for the first 6 months of the study. During the second 6 months there were biweekly in-person visits alternating with bi-weekly telephone contacts. During months 12–24, there were twice-per-month contacts (one in-person visit and one telephone contact). Intervention sessions lasted 45–60 min and included the family support person for part of each session.
The goal of the lifestyle program was to achieve sustained weight losses (≥7% of initial percent overweight) by changing dietary intake and physical activity. This threshold is consistent with weight losses associated with favorable changes in health parameters observed in another randomized controlled study of family-based treatment for severe pediatric obesity (7). Evidence-supported behavior change strategies targeting diet and physical activity were taught and practiced. Family support persons were encouraged to model healthy behaviors and promote healthy home environments as well as to provide positive reinforcement for health behavior change.
Study sample
The cohort for the current report included TODAY participants who had data after 6 months of exposure to the randomized treatment. Of 699 TODAY participants, 595 (85.1%) were included in the analysis. Participants excluded (n = 104) did not differ significantly from the analytic sample on any demographic characteristic, treatment group assignment or baseline percent overweight. Of those excluded, 81 reached the primary outcome before 6 months, and 23 were lost to follow-up or had missing 6 month data. Excluded participants spent a longer time in run-in (98.4 ± 40.3 vs. 79.7 ± 38.5 days; P <.0001), did not lose weight during the run-in period (+1.2 ± 3.9 vs. −1.0 ± 3.7 kg; P <.0001), had higher baseline HbA1c (6.7 ± 0.8 vs. 5.9 ± 0.7%; P <.0001), and a lower baseline c-peptide oDI (0.0017 ± 0.0018 vs. 0.0035 ± 0.0032; P < .0001).
Statistical methods
First, we examined weight change metrics as a function of treatment arm and baseline factors associated with 24-month outcome. Linear mixed models for repeated measures were fit to evaluate changes in percent overweight as a continuous outcome, and generalized estimating equations were used to examine the dichotomized measure (i.e. percent overweight decrease ≥7%). All models included treatment arm, visit (time), the interaction between visit and treatment, and the value of the weight-related measure at baseline. Sex, age at baseline and race-ethnicity also were included as covariates. Other baseline variables considered were percent overweight change during run-in, household highest level of education, total annual income and pubertal stage; only percent overweight change during run-in had a significant effect on percent overweight and was included in the final models. The analyses were repeated for the subsample of participants considered adequately adherent in the lifestyle program arm, defined as ≥75% attendance to the weekly sessions during the initial phase of the program. To evaluate whether age at baseline, sex or race/ethnicity influenced weight change differently across the three treatment arms, subgroup analyses were conducted including appropriate interaction terms.
Next, we evaluated the impact of changes in percent overweight and decreases of ≥7% in percent overweight on HbA1c and other cardiometabolic risk factors using the approaches described earlier. All models included the baseline value of the outcome of interest, treatment arm, sex, age at baseline, race-ethnicity and change in percent overweight during run-in. We also evaluated the potential effects of treatment, sex, age at baseline and race-ethnicity on the association between changes in weight-related measures and corresponding changes in risk factors over time with a series of models including interaction terms for changes in weight-related measures by the factors of interest. Finally, we analyzed the relationship between changes in percent overweight and cardiometabolic risk separately by treatment group.
We used SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC). All analyses were considered exploratory, but because of the number of tests performed, P < .001 was the cut-off for statistical significance.
Results
Sample
Baseline characteristics of the analysis sample (n = 595) are summarized in Table 1. Participants averaged 13.9 years, had an average BMI of 34.8 kg/ m2, and were 78.3% overweight. Average decrease in percent overweight during run-in was 2.6% over an average length of 79.7 days prior to randomization. As per protocol, participants had adequate glycemic control with an average HbA1c of 5.9%. There were no differences in any baseline variable as a function of treatment arm.
Table 1.
Demographic and baseline characteristics (mean ± SD or percent) overall and by treatment group*
| Overall (n = 595) | M (n=193) | M+R (n=201) | M+L (n =201) | |
|---|---|---|---|---|
| Female | 63.5% | 62.7% | 63.7% | 64.2% |
| Age (years) | 13.9 ± 2.0 | 14.0 ± 1.9 | 14.0 ± 2.1 | 13.7 ± 2.1 |
| Race/ethnicity | ||||
| Black non-Hispanic | 30.9% | 29.0% | 28.9% | 34.8% |
| Hispanic | 41.2% | 42.5% | 43.8% | 37.3% |
| White non-Hispanic | 20.5% | 21.8% | 19.4% | 20.4% |
| Other | 7.4% | 6.7% | 8.0% | 7.5% |
| Tanner stage | ||||
| 4–5 | 87.9% | 89.1% | 89.6% | 85.1% |
| <4 | 12.1% | 10.9% | 10.4% | 14.9% |
| Head of household education level | ||||
| 12th grade or less | 26.7% | 27.2% | 26.0% | 26.8% |
| High school graduate, GED, technical degree | 24.1% | 23.0% | 19.9% | 29.3% |
| Some college/associates degree | 32.1% | 33.5% | 36.2% | 26.8% |
| Bachelors degree or higher | 17.1% | 16.2% | 17.9% | 17.2% |
| Household annual income level | ||||
| <$25000 | 41.1% | 38.5% | 42.1% | 42.5% |
| $25000–49 999 | 34.2% | 39.7% | 29.2% | 33.9% |
| >$49 999 | 24.7% | 21.8% | 28.7% | 23.7% |
| Run-in length (days) | 79.7 ± 38.5 | 79.5 ± 29.8 | 82.3 ± 53.3 | 77.4 ± 26.3 |
| Change in percent overweight during run-in (%) | −2.6 ± 7.1 | −2.1 ± 7.3 | −2.6 ± 7.3 | −3.2 ± 6.6 |
| BMI (kg/m2) | 34.8 ± 7.7 | 35.3 ± 7.9 | 35.2 ± 7.8 | 33.8 ± 7.3 |
| BMI standard deviation score (SDS) | 2.2 ± 0.5 | 2.2 ± 0.4 | 2.2 ± 0.5 | 2.2 ± 0.5 |
| Percent overweight (%) | 78.3 ± 37.6 | 80.1 ± 38.1 | 80.2 ± 38.3 | 74.6 ± 36.4 |
| HbA1c (%) | 5.9 ± 0.7 | 6.0 ± 0.7 | 5.9 ± 0.7 | 5.9 ± 0.7 |
| Blood pressure (mm Hg) | ||||
| Systolic | 112.8 ± 11.0 | 113.1 ± 11.2 | 113.3 ± 10.6 | 112.0 ± 11.1 |
| Diastolic | 66.5 ± 8.3 | 66.2 ± 8.4 | 67.2 ± 8.2 | 66.2 ± 8.4 |
| LDL (mmol L−1) | 2.18 ± 0.65 | 2.16 ± 0.65 | 2.20 ± 0.65 | 2.17 ± 0.64 |
| HDL (mmol L−1) | 1.00 ± 0.22 | 0.96 ± 0.22 | 1.02 ± 0.22 | 1.02 ± 0.22 |
| Total cholesterol (mmol L−1) | 3.74 ± 0.76 | 3.70 ± 0.75 | 3.79 ± 0.79 | 3.72 ± 0.75 |
| Triglycerides (mmol L−1) | 1.24 ± 0.81 | 1.29 ± 0.74 | 1.26 ± 0.92 | 1.18 ± 0.76 |
| C-peptide oDI | 0.0035 ± 0.0032 | 0.0032 ± 0.0025 | 0.0036 ± 0.0036 | 0.0036 ± 0.0033 |
The three treatment are the following: metformin monotherapy (M), metformin plus rosiglitazone (M+R), and metformin plus intensive lifestyle (M+L). The P values from generalized linear models testing for baseline treatment differences are all > 0.05.
Changes in weight-related metrics
Changes in percent overweight over time are presented in Table 2 and illustrated in Figure S1A. Changes in BMI are presented in Table S1. Consistent with the TODAY clinical trial, data from the current sample showed that the lifestyle program arm (M+L) was associated with favorable changes in percent overweight and BMI in comparison to M+R at 12 and 24 month assessments (P <.0001), but there were no differences between M and M+L. Results among youth who attended at least 75% of the intensive lifestyle change phase sessions showed the same pattern (Table S2).
Table 2.
Percent overweight and change from baseline (Δ) across study visits by treatment group*
| Percent overweight and Δ |
Pairwise comparison P value |
|||||
|---|---|---|---|---|---|---|
| M | M+R | M+L | M vs. M+R | M vs. M+L | M+R vs. M+L | |
| Baseline | 80.1 ± 38.1 | 80.2 ± 38.3 | 74.6 ± 36.4 | |||
| n at baseline | 193 | 201 | 201 | |||
| Month 6 | 79.2 ± 38.3 | 81.1 ± 39.3 | 70.7 ± 37.4 | |||
| Δ 6-0 | −0.9 ± 8.3 | +0.8 ± 8.9 | −3.9 ± 9.2 | 0.1276 | 0.0069 | <.0001 |
| Month 12 | 76.8 ± 40.0 | 84.1 ± 41.1 | 70.7 ± 38.7 | |||
| Δ 12-0 | −2.0 ± 10.9 | +3.0 ± 12.4 | −4.2 ± 11.6 | <.0001 | 0.1272 | <.0001 |
| Month 24 | 77.4 ± 41.8 | 85.6 ± 38.9 | 72.4 ± 39.7 | |||
| Δ 24-0 | −2.4 ± 14.4 | +4.3 ± 17.4 | −33 ± 14.5 | <.0001 | 0.6409 | <.0001 |
Mean ± SD are presented. The three treatment groups are the following: metformin monotherapy (M), metformin plus rosiglitazone (M+R), and metformin plus intensive lifestyle (M+L). The P values were calculated from repeated measures models testing for Pairwise treatment differences in percent overweight change during the first 2 years of the study. The P values presented are from adjusted models for baseline percent overweight, time, treatment, sex, age at baseline, race/ethnicity, percent overweight change during run-in, and an interaction term for time-by-treatment.
Figure S1B illustrates the impact of lifestyle program on achieving the a priori weight loss goal of a decrease of at least 7% in percent overweight from baseline. As shown, more than 30% of those randomized to M+L decreased ≥7% at each assessment point, but there were no significant differences between M and M+L participants in achieving threshold levels of weight loss. Results were the same when M+L participants with adequate lifestyle program attendance were considered (Figure S2).
Subgroup analyses
We examined whether sex, age at baseline or race-ethnicity were related to weight change across the three treatment arms. There was a main effect of sex for change in percent overweight and decrease of >7% in percent overweight. Specifically, percent overweight increased +0.2% ± 12.2 in females and decreased by −2.9% ± 11.9 in males (P = 0.0001) over time. Similarly, 23.8% of females vs. 32.3% of males decreased percent overweight by at least 7% (P = 0.0003). However, no sex by treatment interactions were observed (Tables S3 and S4). Neither race-ethnicity nor age at baseline was associated with changes in weight metrics (data not shown).
Relation of changes in weight metrics and cardiometabolic measures
We examined whether changes in percent overweight over time were related to changes in cardiometabolic outcomes adjusting for treatment arm and other conceptually relevant variables (Table S5). There was a linear relationship between changes in cardiometabolic outcomes (except diastolic blood pressure) as a function of changes in percent overweight. For example, for each one unit decrease in percent overweight, there was a corresponding decrease in HbA1c of .0136%. Similarly, for each decrease in percent overweight there were corresponding decreases in systolic blood pressure, low-density lipoprotein cholesterol, triglycerides and total cholesterol, and increases in high-density lipoprotein cholesterol and c-peptide oDI. There were no interactions between percent overweight changes and treatment (Table S6), sex, race-ethnicity or age at baseline for any of the cardiometabolic outcomes of interest.
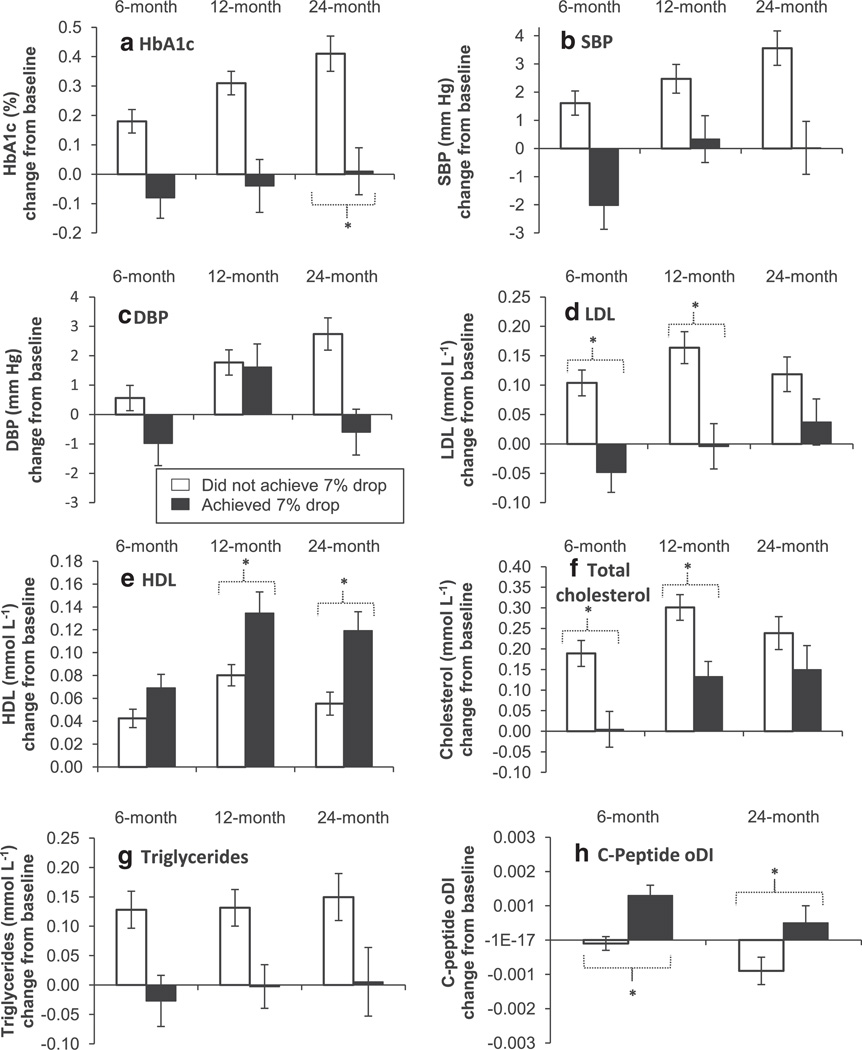
We next evaluated the impact of a decrease in percent overweight of at least 7% over time (Fig. 1A – H). Overall, the pattern of results shows deterioration in cardiometabolic risk factors in youth who did not lose at least 7% of percent overweight and stability or improvement in risk factors in those who lost at least 7%. The benefits of a 7% decrease relative to smaller decreases (or increases) were seen for all measures assessed. Moreover, significant between-group differences in HbA1c, HDL and C-Peptide oDI were sustained at month 24.
Figure 1.
Multi-panel figure of change from baseline (mean and SE bars) in cardiometabolic outcome (hemoglobin A1c [HbA1c] (A); systolic blood pressure [SBP] (B); diastolic blood pressure [DBP] (C); low-density lipoprotein LDL cholesterol (D); high-density lipoprotein (HDL) cholesterol (E); total cholesterol (F); triglycerides (G); and c-peptide oral disposition index [oDI] (H) at month 6, 12 and 24, separately for youth who achieved a drop of ≥7% in percent overweight since baseline vs. those who did not. The ‘*’ indicates a significant difference (P < .001) between the two groups at a particular time point, based on GEE repeated-measures models adjusted for the baseline value of the cardiometabolic outcome, visit, treatment, sex, race-ethnicity, age at baseline and change in percent overweight during run-in. C-peptide oDI based on OGTT data not available at month 12 per study protocol.
Discussion
Results of the present analysis confirm that although the TODAY intensive lifestyle program was associated with modest decreases in proxies of adiposity over time, it did not confer a weight change advantage over treatment with metformin alone for the total sample or any sub-group. However, the current findings provide compelling evidence that weight loss is an important goal for youth with T2DM as changes in weight metrics were associated with significant improvements in cardiometabolic risk factors across treatment arms.
We reported previously that the addition of a comprehensive lifestyle intervention to treatment with metformin alone did not improve sustained glycemic control or maintain early decreases in overweight in youth with T2DM (1). In the present analysis, we examined changes in weight-related measures in a more a detailed examination of the sub-sample of 595 TODAY participants who completed the first 6 months of the randomized controlled trial. The analysis of weight-related outcomes for participants with full exposure to the weekly phase of intervention showed that there were no significant differences between M and M+L treatment arms in any weight-related measure over time. Moreover, no predictors of success emerged. Adequate attendance (at least 75% of sessions) was not associated with larger decreases in percent overweight. Subgroup analysis showed that although boys were more likely than girls to decrease in percent overweight and to show a decrease in overweight of at least 7% over time, there was no evidence that sex was related differentially to changes in weight metrics as a function of treatment arm. Finally, neither age at baseline nor race-ethnicity was associated with changes in weight-related measures. Thus, the current results provide no evidence that lifestyle intervention was more or less beneficial for any particular subgroup of TODAY participants.
Although lifestyle intervention did not enhance outcomes, we deemed that it of critical importance to examine the impact of weight changes on cardiometabolic outcomes across treatment arms to evaluate whether weight control should be a treatment goal for youth with T2DM. Our analysis documented a small, but significant dose–response relationship between changes in percent overweight and each of the cardiometabolic outcomes examined, with the exception of diastolic blood pressure. For each 1% decrease in percent overweight there was a corresponding decrease of .0136% in HbA1c after controlling for multiple covariates. Thus, youth who achieved the preplanned goal of a decrease of at least 7% in percent overweight saw a corresponding decrease in HbA1c of .0952%, a decrease considered to be clinically meaningful in adults with T2DM (8). Although the clinical significance of other observed changes in risk factors in response to changes in percent overweight is less clear, we argue that the current findings provide important information given increasing hypertension and dyslipidemia in the TODAY cohort over time, independent of treatment and despite expert clinical management (9,10).
Notably, the benefits of decreases in percent overweight did not vary as a function of treatment, sex, age at baseline or race-ethnicity, documenting the importance of weight decreases for all youth with T2DM. A comparison of cardiometabolic outcomes in participants who did and did not meet the 7% threshold level decrease in percent overweight further documented the positive effects of weight loss on outcomes over time, with benefits sustained for HbA1c, HDL and C-peptide oDI over a two-year period. Given the benefits of decreases in percent overweight and worsening of risk associated with increases in percent overweight, the current findings provide convincing evidence that weight management is a crucial goal for youth with T2DM.
It is important to consider factors specific to the TODAY lifestyle intervention before concluding that lifestyle programs have no benefits for adolescents with T2DM. First, TODAY youth participated in a run-in period that involved delivery of comprehensive diabetes education, which encouraged lifestyle changes to promote weight management (11). Thus, all TODAY participants were introduced to the basics of lifestyle intervention, and weight loss of participants in the current study during the 2–6 month run-in period averaged 1.0 ± 3.7 kg. Further, all TODAY participants took metformin, which has been shown to have modest effects on weight in youth with severe obesity aged 8–18 years with hyperinsulinemia or impaired glucose tolerance (12). These factors may have attenuated the impact of the TODAY lifestyle program relative to treatment with metformin alone.
In evaluating the outcomes of the TODAY lifestyle program, it is also instructive to compare findings to those from similar interventions with adolescents without T2DM. The average BMI of TODAY participants was 34.8 kg/m2, and thus the most pertinent studies for comparison are those that have focused on adolescents with more severe obesity (defined as BMI ≥120% of the 95th percentile for age and sex or an absolute BMI ≥35 kg/m2) (5). An examination of these studies reveals that weight losses have been disappointing (13), with longer-term differences observed in one randomized trial explained by larger weight increases in the control group (14). Indeed, behavioral lifestyle interventions appear to be relatively ineffective for older youth with severe obesity (15–18). Thus, the modest decreases in percent overweight and BMI (BMI changes of −.2, +.2 and +1.4 at months 6, 12, and 24) observed in the TODAY lifestyle program across 2 years of follow-up are not dissimilar to those observed in other investigations focusing on adolescents with severe obesity.
There is growing consensus that aggressive treatment of T2DM and its comorbidities in youth is indicated (19,20). Given the present evidence of the health benefits of weight loss, and accumulating evidence that current lifestyle interventions have limited impact on adolescents with severe obesity, there is an imperative to identify and evaluate strategies to optimize the impact of obesity interventions and promote weight control in youth with T2DM. The evaluation of novel approaches that integrate lifestyle interventions with diabetes management, different dietary strategies, medications and bariatric surgery is needed because youth with T2DM require sustained intervention to address developmental and medical needs (21).
Supplementary Material
Acknowledgments
The TODAY Study Group gratefully acknowledges the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma and Oklahoma City Area Indian Health Service. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the respective Tribal and Indian Health Service Institution Review Boards or their members. Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu/.
Funding support
This work was completed with funding from National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health grant numbers U01-DK-061212, U01-DK-061230, U01-DK-061239, U01-DK-061242 and U01-DK-061254, from the National Center for Research Resources General Clinical Research Centers Program grant numbers M01-RR-000036 (Washington University School of Medicine), M01-RR-000043-45 (Children’s Hospital Los Angeles), M01-RR-000069 (University of Colorado Denver), M01-RR-000084 (Children’s Hospital of Pittsburgh), M01-RR-001066 (Massachusetts General Hospital), M01-RR-000125 (Yale University) and M01-RR-014467 (University of Oklahoma Health Sciences Center), and from National Center for Research Resources Clinical and Translational Science Awards grant numbers UL1-RR-024134 (Children’s Hospital of Philadelphia), UL1-RR-024139 (Yale University), UL1-RR-024153 (Children’s Hospital of Pittsburgh), UL1-RR-024989 (Case Western Reserve University), UL1-RR-024992 (Washington University in St. Louis), UL1-RR-025758 (Massachusetts General Hospital) and UL1-RR-025780 (University of Colorado Denver).
Abbreviations
- T2DM
type 2 diabetes mellitus
- TODAY
Treatment Options for type 2 Diabetes in Adolescents and Youth
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- M
metformin
- M+R
metformin plus rosiglitazone
- M+L
metformin plus an intensive lifestyle program
- HbA1c
hemoglobin A1c
- BMI
body mass index
- OGTT
oral glucose tolerance test
- oDI
oral disposition index
- 1/IF
1/fasting insulin
- ΔC30/ΔG30
c-peptide index (defined as the ratio of the incremental c-peptide and glucose responses over the first 30 min of the OGTT test)
- LDL
low-density lipoprotein cholesterol
- HDL
high-density lipoprotein cholesterol
Footnotes
Trial Registration: ClinicalTrials.gov NCT00081328
Conflicts of interest
The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, LifeScan, Inc., Pfizer, and Sanofi-Aventis. MDM is a member of the Scientific Advisory Board of Weight Watchers International, Inc. DEW is a consultant for Shire Pharmaceuticals. PZ is a consultant for Daiichi-Sankyo, Astra-Zeneca, Merck, Takeda, and Eli Lilly. NWA is a consultant for Daiichi-Sankyo. LE, BL, KH, CEIL and DJB have nothing to disclose.
Author contributions
The writing group was composed of Marsha D Marcus (chair), Denise E Wilfley, Laure El ghormli, Philip Zeitler, Barbara Linder, Kathryn Hirst, Carolyn E Ievers-Landis, Dorothy J van Buren and Natalie Walders-Abramson. A complete list of the members of the TODAY Study Group can be found in the Supplementary Data online. MDM and KH researched data, wrote the manuscript, reviewed/edited the manuscript and contributed to the discussion. DEW, BL, CEIL and DJB reviewed/edited the manuscript and contributed to the discussion. LE performed the analysis, researched data and reviewed/edited parts of the manuscript. PZ researched data, reviewed/edited the manuscript and contributed to the discussion. NWA wrote the manuscript, reviewed/edited the manuscript and contributed to the discussion. All authors had final approval of the submitted and published version.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Figure S1. Change from baseline in percent overweight (A) and percent of youth who achieved a drop ≥7% since baseline (B) across study visits (at 6, 12 and 24 months) by treatment group*.
Figure S2. Percent (%) of youth who achieved a drop ≥7% because baseline across study visits, by treatment group, among youth in the M+L group who attended at least 75% of the intensive lifestyle change phase sessions*.
Table S1. BMI and change from baseline (Δ) across study visits by treatment group*.
Table S2. Percent overweight and change from baseline (Δ) across study visits, among youth in the M+L group who attended at least 75% of the intensive lifestyle change phase sessions, by treatment group*.
Table S3. Descriptive statistics for percent overweight and change from baseline (Δ) across study visits, by treatment group and sex.
Table S4. Descriptive statistics for percent (%) of youth who achieved a ≥7% drop in percent overweight since baseline across study visits, by treatment group and sex.
Table S5. Association between changes in percent overweight and cardiometabolic outcomes during the first 2 years of the study*.
Table S6. Descriptive statistics for cardiometabolic outcomes and change from baseline (Δ) for all visits combined, by treatment group and by percent (%) of youth who achieved a ≥7% drop in percent overweight since baseline.
References
- 1.Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. 2013;36:1749–1757. doi: 10.2337/dc12-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Study Research Group TODAY. Zeitler P, Epstein L, Grey M, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2000;246:1–190. [PubMed] [Google Scholar]
- 5.Flegal KM, Wei R, Ogden CL, et al. Characterizing extreme values of body mass index-for-age by using the 2000 centers for disease control and prevention growth charts. Am J Clin Nutr. 2009;90:1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 6.TODAY Study Research Group. Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. Int J Obes (Lond) 2010;34:217–226. doi: 10.1038/ijo.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalarchian MA, Levine MD, Arslanian SA, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124:1060–1068. doi: 10.1542/peds.2008-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.TODAY Study Group. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1735–1741. doi: 10.2337/dc12-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TODAY Study Group. Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1758–1764. doi: 10.2337/dc12-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grey M, Schreiner B, Pyle L. Development of a diabetes education program for youth with type 2 diabetes. Diabetes Educ. 2009;35:108–116. doi: 10.1177/0145721708325156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendall D, Vail A, Amin R, et al. Metformin in obese children and adolescents: the MOCA trial. J Clin Endocrinol Metab. 2013;98:322–329. doi: 10.1210/jc.2012-2710. [DOI] [PubMed] [Google Scholar]
- 13.Luca P, Dettmer E, Khoury M, et al. Adolescents with severe obesity: outcomes of participation in an intensive obesity management programme. Pediatr Obes. 2015;10:275–282. doi: 10.1111/ijpo.261. [DOI] [PubMed] [Google Scholar]
- 14.Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 15.Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolesc Med. 2012;166:1103–1108. doi: 10.1001/2013.jamapediatrics.319. [DOI] [PubMed] [Google Scholar]
- 16.Johnston CA, Tyler C, Palcic JL, Stansberry SA, Gallagher MR, Foreyt JP. Smaller weight changes in standardized body mass index in response to treatment as weight classification increases. J Pediatr. 2011;158:624–627. doi: 10.1016/j.jpeds.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 17.Knop E, Singer V, Uysal Y, et al. Extremely obese children respond better than extremely obese adolescents to lifestyle interventions. Pediatr Obes. 2015;10:7–14. doi: 10.1111/j.2047-6310.2013.00212.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwimmer JB. Clinical trials for adolescent obesity: cooking up an alphabet stew of what to do. JAMA Pediatr. 2013;167:391–393. doi: 10.1001/jamapediatrics.2013.1661. [DOI] [PubMed] [Google Scholar]
- 19.Linder BL, Fradkin JE, Rodgers GP. The TODAY study: an NIH perspective on its implications for research. Diabetes Care. 2013;36:1775–1776. doi: 10.2337/dc13-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863–3869. doi: 10.2337/dc12-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Springer SC, Silverstein J, Copeland K, et al. American academy of pediatrics: management of type 2 diabetes mellitus in children and adolescents. Pediatrics. 2013;131:e648–e664. doi: 10.1542/peds.2012-3496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.