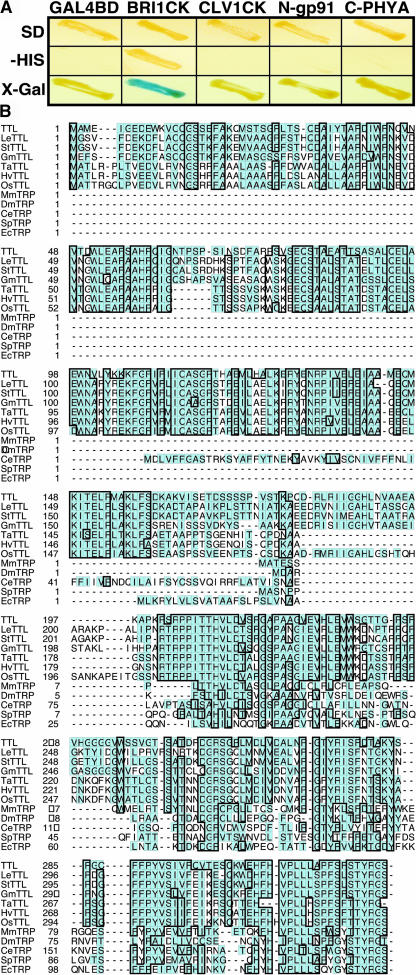
Figure 1.
Identification of TTL as a BRI1-Interacting Protein by Yeast Two-Hybrid Screening.
(A) TTL showed specific interaction with BRI1 kinase domain (BRI1CK), examined by the growth on His-lacking synthetic media and the production of blue colonies on the 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal)–containing media, compared with its interactions with nonrelevant baits, including CLV1 kinase domain (CLV1CK), the N-terminal portion of an Arabidopsis NADPH oxidase (N-gp91), the C-terminal segment of the Arabidopsis phytochrome A (C-PHYA), and the Gal4 binding domain (GAL4BD) alone.
(B) Amino acid sequence alignment of TTL and its homologs from various organisms, including LeTTL from Lycopersicon esculentum, StTTL from Solanum tuberosum, GmTTL from Glycine max, TaTTL from Triticum aestivum, HvTTL from Hordeum vulgare, OsTTL from Oriza sativa, MmTRP from Mus musculus, DmTRP from Drosophila melanogaster, CeTRP from Caenorhabditis elegans, SpTRP from Schizosaccharomyces pombe, and EcTRP from Escherichia coli. Except TTL, other full-length plant TTL sequences were deduced from overlapping EST sequences as follows: LeTTL (AW649495, BE432987, NM535270, and BF114284), StTTL (BE920936, BM405599, and BE919514), GmTTL (AW100149, BE022630, BI468680, and BE824466), TaTTL (BE400327, BQ245413, and BE444381), HvTTL (BE602694, BU989926, and AL503126), and OsTTL (AC092075, AU067993, and CB214315). Sequence alignment was conducted using ClustalW software.