Abstract
Using an eyelid conditioning paradigm modeled after that developed by Little, Lipsitt, and Rovee-Collier (1984), Fifer et al. (2010) demonstrated that newborn infants learn during sleep. This study examined the role of sleep state in neonatal learning. We recorded electroencephalogram (EEG), respiratory, and cardiovascular activity from sleeping full term newborn infants during delay eyelid conditioning. In the experimental group (n = 21), a tone was paired with an air puff to the eye. Consistent with Fifer et al. (2010), newborn infants reliably learned during sleep. The experimental group more than doubled EMR rates to a tone alone, while a control group (n = 17) presented with unpaired tones and puffs maintained low EMR rates. Infant learners were more likely to produce a conditioned EMR during quiet sleep compared to active sleep. Understanding the influence of sleep state on conditioned responses will inform the potential use of eyelid conditioning for early screening.
Keywords: associative learning, electrophysiology, eyeblink conditioning, infant, neonate, sleep, sleep state
1 | INTRODUCTION
Pioneering work by Rovee-Collier and colleagues facilitated a paradigm shift for investigation into the roots of learning and memory (Rovee-Collier & Fagen, 1981; Rovee-Collier & Lipsitt, 1982), demonstrating robust capacities for associative learning and memory retention in young infants. Several studies have confirmed that awake infants are capable of classical conditioning and operant learning even in the first few days of life (DeCasper & Fifer, 1980; Lipsitt, 1998; Papousek, 1961; Siqueland & Lipsitt, 1966; Sullivan et al., 1991). Both positive approach behaviors and avoidance behaviors can be shaped in waking neonates by reinforcing neonatal reflexes (Lipsitt, 1998). The brain is immature at birth, and rapidly developing neural architecture is influenced by experience (Black, 1998). Young infants’ early emerging learning capacity, established by this seminal work by Rovee-Collier and others, represents a mechanism through which awake infants are able to incorporate information about their environment to shape brain development and guide their own adaptive behavior.
In recent years, there has been growing interest in understanding the role of sleep in neonatal learning and memory, given that newborns spend over 70% of their time asleep and can only maintain wakefulness for brief intervals (Anders, Sadeh, & Appareddy, 1995; So, Adamson, & Horne, 2007). Sleep facilitates neural maturation and memory consolidation processing; moreover, infants may also be able to learn and adapt to the environment even while asleep (see for review, Tarullo, Balsam, & Fifer, 2011). Neonates process information while asleep. For example, they actively respond to auditory and visual stimuli while asleep (Cheour et al., 2002; Cruz, Crego, Ribeiro, Goncalves, & Sampaio, 2015; deRegnier, Nelson, Thomas, Wewerka, & Georgieff, 2000; Kotilahti et al., 2010; Sambeth, Ruohio, Alku, Fellman, & Huotilainen, 2008). Event related potential (ERP) studies show a differential response to mother’s voice versus a stranger’s voice (deRegnier et al., 2000) and a mis-match response to fearful versus angry voices (Zhang et al., 2014). Near-infrared spectroscopy (NIRS) has been used to demonstrate hemodynamic responses to speech and music in sleeping newborns (Kotilahti et al., 2010). Thus, even during sleep, neonates are processing sensory inputs and integrating information about their environments.
Previously, we demonstrated learning in neonates when information was presented only during sleep (Fifer et al., 2010). In that study, sleeping neonates underwent a delay eyeblink conditioning protocol in which a tone was paired with an air puff to the eye. Delay eyelid conditioning is widely used in the animal literature (e.g., Clark, Zhang, & Lavond, 1992; Ivkovich & Stanton, 2001). Moreover, awake human infants reliably acquire a conditioned response (Herbert, Eckerman, & Stanton, 2003; Ivkovich, Collins, Eckerman, Krasnegor, & Stanton, 1999), even within the neonatal period (Little et al., 1984). When we administered a delay eyelid conditioning paradigm to sleeping neonates, 24 out of 26 sleeping neonates in the first days of life exhibited their ability to learn by increasing their rate of conditioned eye movements in response to the tone alone within a single session (Fifer et al., 2010). Furthermore, none of the four control infants, who experienced random pairings of the tone and puff, evidenced an increase in eye movement responses (EMRs) to the tone alone. This strongly suggests that the increase in eye movement responses in the experimental group was due to learning. In that first study, the small number of infants in the control group precluded comparison of the trajectories of EMRs over the course of the session in the experimental versus control groups. It was clear that infants reliably acquired the conditioned response within the half-hour conditioning session, but it would be informative to examine how quickly infants began to show signs of learning.
Even while asleep, infants are surrounded by environmental contingencies, often spanning multiple sensory modalities. However, little is known about what conditions may be optimal for infant learning during sleep. One unique characteristic of neonatal sleep is rapid cycling between quiet (non-REM) and active (REM) sleep states (Anders et al., 1995; Mirmiran, Maas, & Ariagno, 2003). Prechtl (1974) described both quiet and active sleep states in the neonatal period, with quiet sleep characterized by regular respiration and heart rate, while active sleep is characterized by variable respiration and heart rate. In Fifer et al. (2010), though infants in both groups appeared to be asleep during the experimental protocol, sleep state was not monitored. Animal studies have suggested that classical conditioning may be influenced by sleep state. Rodents in a classical conditioning paradigm during REM sleep were able to learn a conditioned response (Hennevin, Hars, Maho, & Bloch, 1995) though no learning appeared to occur during non-REM sleep. Using a mismatch negativity paradigm, differential reactivity to fearful versus angry voices was observed during active sleep in newborns (Zhang et al., 2014). Recently, Barnes and Wilson (2014) showed that olfactory memories could be enhanced or disrupted in rodents and that the effect was confined to slow wave sleep. This led us to consider whether classical conditioning during sleep could be state dependent for human infants as well. Infants assessed with the delay eyelid conditioning paradigm will vary in whether they are in active sleep, quiet sleep, or some combination thereof. If acquisition or production of a conditioned response is state dependent, this variation in sleep state during the session could contribute to observed individual differences in neonatal learning during sleep.
In the current study, a single session of delay eyelid conditioning was administered to sleeping human neonates, and a control group of neonates was presented with the same tone and puff stimuli but in an unpaired quasi-random sequence. EMRs were monitored via electroencephalogram (EEG) and cardiovascular and respiratory activity was recorded to assess sleep state. Our first goal was to replicate the finding of Fifer et al. in another cohort of infants with equal numbers of control and experimental subjects, enabling us to examine how quickly behavioral signs of learning would be evidence in the experimental group as compared to the control group. The second goal was to examine whether conditioned responses are more likely to occur in active versus quiet sleep.
2 | METHODS
2.1 | Participants
Healthy full term infants (N = 45) were recruited from the newborn nursery of Morgan Stanley Children’s Hospital of New York at Columbia University Medical Center, and tested during sleep in either the experimental or control condition. Participants had a gestational age of 38–41 weeks (M = 39.84, SD = .75) and were tested within 3 days of birth (M = 31.79 hr; SD = 15.56 hr). All infants had passed the hearing test that was routinely administered by hospital staff and had a mother fluent in English or Spanish, the languages in which research staff were qualified to administer informed consent. Exclusion criteria included major medical problems. Two infants in the experimental group were excluded from analyses due to technical difficulties. In the control group, three infants were excluded due to technical difficulties; one due to fussiness; and one because the mother chose to withdraw during the study. Thus, there were 38 infants included in the current analyses (experimental group, N = 21, nine male; control group, N = 17, six male). Independent samples t-tests and chi-squared tests verified that the experimental and control groups did not differ in birthweight, method of delivery (C-section or vaginal), gestational age at birth, age at testing, maternal age, sex, or ethnicity.
2.2 | Procedures
All procedures used in this study were approved by the Institutional Review Boards at the New York State Psychiatric Institute and the Columbia University Medical Center. Attending physicians and nurses on the unit assisted in identifying infants who met criteria for the study. Research staff then approached mothers to describe the study and obtain informed consent. Infants were tested while asleep, in a quiet room within the newborn unit. Infants were fed immediately prior to testing to increase the likelihood that they would remain asleep during the session. After being fitted with three heart rate electrodes, a respiratory inductance cloth belt (Ambulatory Monitoring Inc., Ardsley, NY), and a 124-electrode sensor net (Electrical Geodesics Inc., Eugene, OR; EGI), the infant was swaddled and placed supine in a bassinet cushioned with a Tempur-Pedic pillow to reduce any pressure points from the electrodes. Electrode impedances were measured and adjustments were made until impedance was below 50 kWΩ. Research staff then waited until the infant was asleep before beginning the testing session. A flexible tube was configured to deliver a puff of air (.05 psi) to the outer canthus of the right eye using an air puff unit (San Diego Instruments, San Diego, CA). An experimenter continuously monitored the position of the infant’s head during the study and adjusted the position of the flexible tube as needed if the infant moved, so as to maintain a consistent location and angle for the delivery of the air puff to the outer canthus of the right eye. Tones were presented using two speakers with padded headphones, positioned in the bassinet directly next to the ears (headphones were about half inch from the ears, allowing room for the EEG net), using rolled towels for consistent positioning relative to the size of the each infant’s head. During the testing session, EEG and cardiorespiratory data were recorded while presenting the sleeping infant with tones bilaterally through the speakers and puffs of air to the right eye. EEG was recorded to a vertex reference and data were sampled from all channels at 1,000 Hz. Heart rate variability and respiratory patterns, as well as experimental observational notes, were used to confirm that infants remained asleep through the study. EEG data were later scored for eye movement responses (EMR), and cardiorespiratory data were later scored for sleep state.
2.3 | Delay eye movement response (EMR) conditioning paradigm
The EMR conditioning procedure, modeled after the standard delay eyeblink conditioning procedure used with awake infants, was administered in a single 32 min session, following the methods of Fifer et al. (2010). All infants were first presented with two tone-alone trials and two puff-alone trials. The experimental group was then presented with 20 blocks of 10 trials. Each block of 10 trials consisted of eight paired tone-puff trials, in which a 1,000 ms, 1,000 Hz tone preceded and co-terminated with a 100 ms air puff to the right eye; one puff-alone trial; and one tone-alone trial. The puff-alone trials allowed for monitoring of the unconditioned response over the course of the session, and the tone-alone trials were the test trials to assess conditioned responding without the interference of the air puff eliciting an unconditioned response. Tones were ~80 dB at the position of the infant’s ears, from speakers positioned The sequence of trials in each block was five paired tone-puff trials followed by the puff-alone trial, three more paired tone-puff trials, and then a tone-alone trial. The inter-trial intervals varied from 6 to 10 s to prevent temporal entrainment, with an average of 8 s. The control group was presented with the same number of tone and puff stimuli over the same duration, but in a quasi-random sequence such that the tone and puff were never paired. For the control group, stimulus presentations were separated by a variable interval ranging from .5 to 8.5 s (mean of 4.5 s). The order of presentation of tones and puffs was chosen using a Gellermann series (Gellermann, 1933) such that: (i) the sequence of stimuli did not contain more than three consecutive presentations of a single type of stimulus (tone or puff) and (ii) no block of stimuli contained more than nine changes in stimulus type on consecutive trials.
2.4 | Eye movement responses
Offline, eye movement responses were scored from electrical potentials near the eyes using Matlab software (MathWorks, Natick, MA). EEG data were notch filtered at 60 Hz and a .5 Hz highpass filter was applied. Data were then segmented with respect to the tone alone trials to visually inspect the 900–2,000 ms following tone onset for evidence of eye movement responses (EMRs), consistent with Fifer et al. (2010). Puff alone trials were scored first, to verify that the unconditioned stimulus consistently elicited an eye movement response (EMR) for all infants in both groups. For the experimental group, the 20 tone-alone trials (one in each block) were then examined. For the control group, all tone presentations were tone-alone trials, so the last tone-alone trial in each block was scored so as to compare an equal number of tone-alone trials in the two groups. The trials were presented in a random scrambled order, different for each participant, so that as coders were scoring the trials, they were blind to when in the session the trial had occurred.
For each trial, an automatic artifact rejection algorithm marked as bad any channels with excessively noisy data or zero variance. For tone alone trials, a montage of 10 bipolar channels near the eye was scored for EMRs, defined as deviations of at least 20 μV occurring in the 900–2,000 ms after tone onset, indicating ocular activity. The use of bipolar channels, in which two adjacent electrodes both located near the eyes were referenced to each other, facilitated clear visual detection of EMRs. The channel pairs were: E33-E26; E26-E22; E22-E23; E22-E18; E22-E17; E17-E14; E14-E15; E14-E9; E14-E8; and E8-E1. Upon identifying potential EMRs in the bipolar montage, a global view of all 124 channels referenced to the vertex, also for the 900–2,000 ms after tone onset, was used to verify that the deviations observed in the bipolar montage were most pronounced in frontal channels, consistent with ocular activity as opposed to gross muscle movements or other distortions of the EEG. Trials contaminated with artifact were excluded from analysis, and the valid tone alone trials were scored as 0 or 1 based on the presence or absence of an EMR. As in Fifer et al., 2010, blocks were collapsed into five epochs of four blocks (40 trials) each, and tone-alone EMRs were averaged within these epochs for purposes of examining response trajectories.
2.5 | Sleep state
Sleep state was monitored during the procedure and then visually coded based on variability in the respiratory tracing (Haddad, Jeng, Lai, & Mellins, 1987; Harper, Schechtman, & Kluge, 1987). The respiratory tracings were displayed and the peak inspiratory value for each breath was marked using specially designed software. After visual verification of these marks, the instantaneous breathing rate (IBR) was computed, displayed, and visually coded into high and low variability states corresponding to periods of quiet and active sleep. Periods of wakefulness were identified based on respiratory tracings and IBR in conjunction with study notes. Four infants in the experimental group and one in the control group were missing sleep state data due to technical difficulties.
3 | RESULTS
3.1 | Conditioning
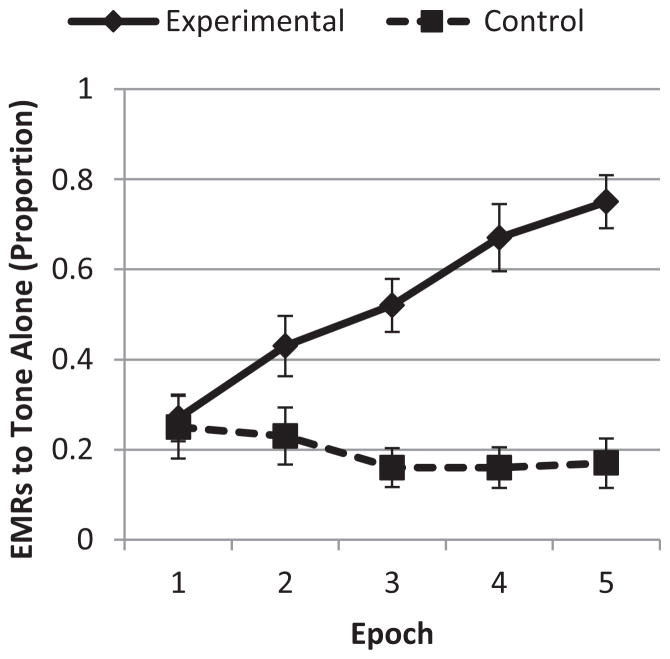
EMR rates were examined for Tone Alone trials. As shown in Figure 1, the experimental group had a .75 rate of conditioned EMRs in the last epoch of the session, as compared to a .27 EMR rate in the first epoch. The control group, in contrast, had a .17 EMR rate in the last epoch, down from .25 in the first epoch. Thus, while the two groups began with a similar EMR rate, the experimental group showed a steep increase in EMRs across the conditioning session, whereas the control group maintained a low rate of responding throughout the session. To examine group differences in EMR rates to the Tone Alone trials, a Repeated Measures Analysis of Variance (RM ANOVA) was conducted with epoch as the within subjects factor and group as the between subjects factor. There were main effects of group, F(1, 33) = 44.10, P < .001, partial η2 = .572, and epoch, F(4, 132) = 3.97, P = .004, partial η2 = .107, as well as an epoch × group interaction, F(4, 132) = 6.96, P < .001, partial η2 = .174. Analyses to follow up the epoch × group interaction showed a significant increase in EMR across epochs for the experimental group, but not for the control group. The trajectories of EMR rates quickly began to diverge: As early in the session as epoch 2, the experimental group had a higher response rate (P = .03), and the groups differed significantly in their EMR rates for all epochs except for the first one (P’s < .001 for epochs 3–5).
FIGURE 1.
Change in eye movement responses by group. Bars indicate SE
The experimental group more than doubled their EMR rate from epoch 1 to epoch 5, with a mean increase of .46 (SD.27). The control group rate of responding remained flat across the session (M change = −.07, SD = .31). Within the experimental group, learners were defined as having at least 50% EMRs in epoch 5 as well as an increase in rate of EMRs from the 1 to 5 epoch, determined by subtracting EMR rate in epoch 1 from EMR rate in epoch 5. Of the 21 infants in the experimental group, 19 were classified as learners. Of 17 infants in the control group, two would have met the criteria for learners. The infants in the experimental group were far more likely to be classified as learners, χ2(1, 38) = 23.54, P < .001, Cramer’s V = .787, providing clear evidence that this pattern of EMRs reflected the acquisition of a conditioned response to the tone-puff pairing.
To ascertain if demographic or birth characteristics accounted for individual variations in EMRs within the experimental group, stepwise regressions were conducted. EMR rate in epoch 5 and the change score from epoch 1 to epoch 5 were separately regressed on birthweight, method of delivery (C-section or vaginal), gestational age, age at testing, sex, ethnicity, and maternal age. No predictors were entered in either model, indicating that these demographic and birth characteristics did not account for individual variations in conditioning in the current sample.
3.2 | Sleep state
Active sleep was the predominant behavioral state during the conditioning paradigm. In the first half of the session, the distribution of behavioral states in the experimental group was 65.34% active sleep, 31.25% quiet sleep, and 3.41% awake. In the second half of the session, once a conditioned response had been acquired, the distribution of behavioral states was similar: 66.35% active sleep, 24.94% quiet sleep, and 8.71% awake. Paired sample t tests comparing the percentage of each behavioral state observed in the experimental group during the first versus second halves showed that the distribution of behavioral states did not change significantly over the course of the conditioning session. While a few infants remained in active sleep for the entire session, most transitioned between behavioral states during the 30 min conditioning paradigm, consistent with the frequent state transitions characteristic of the neonatal period (Mirmiran et al., 2003). Paired sample t tests were also used to compare the experimental and control groups on the percentage of active sleep, quiet sleep, and awake states, separately for each half. There were no significant differences between groups.
For the experimental group, to examine whether the likelihood of observing an EMR during a tone alone trial varied as a function of what sleep state the infant was in during the trial, a logistic regression model was conducted with trial as the unit of analysis, using bias-corrected bootstrapping with 5,000 samples (Hayes, 2013). Only data from tone-alone trials of learners in the experimental group were included, as the focus was on determining how sleep state related to observation of a conditioned response. Four of the nineteen learners were missing autonomic data due to technical difficulties, thus 15 learners in the experimental group contributed to this analysis. Of 300 possible trials (15 learners × 20 tone-alone trials each), trials were excluded if the infant was awake for any part of the trial or autonomic data were missing for that trial (10%, N = 30), or if EMR data were unavailable due to artifact (14%, N = 42), yielding a final N of 228 trials for the analysis. Sleep state (quiet or active) was the independent variable, EMR (present or absent) was the dependent variable, and session half (first or second) was entered as a dichotomous moderator variable in the model.
As shown in Table 1, there was a main effect of half in predicting EMR, such that EMRs were more likely in the second half of the session, with the 95% confidence interval excluding zero, CI: 3.09–8.29. There was also a main effect of sleep state, CI: 1.21–5.25. Finally, there was a significant interaction between session half and sleep state in predicting EMR, CI: −3.85 to −1.01.
TABLE 1.
Logistic regression model: session half moderates the effect of sleep state on observed EMRs
| Coeff. | SE | Z | P | |
|---|---|---|---|---|
| Intercept | −7.77 | 1.86 | −4.18 | <.001 |
| Session half | 5.69 | 1.33 | 4.29 | <.001 |
| Sleep state | 3.23 | 1.03 | 3.13 | .002 |
| Session half × sleep state | −2.43 | .72 | −3.35 | .001 |
Model fit: −2 LL = 272.48, Cox & Snell R2 = .17
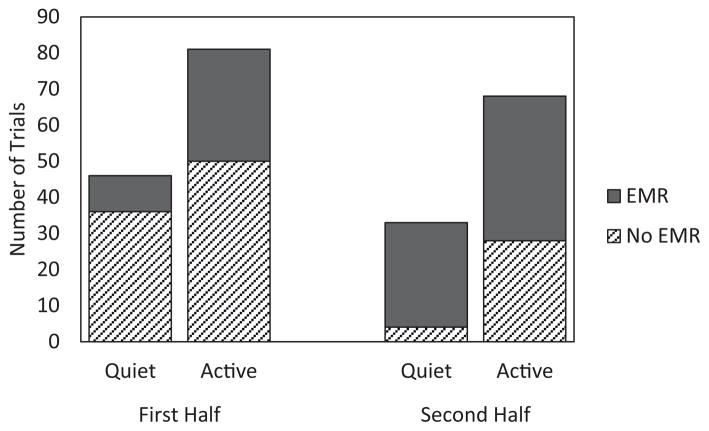
This moderation effect was followed up with χ2 tests examining the association between sleep state and EMR separately for each half of the session. In the first half, there was no significant association between sleep state and EMR, χ2(1, 127) = 3.67, P = .06, Cramer’s V = .17. In the second half, however, EMRs were disproportionately likely to be observed during quiet sleep, χ2(1, 101) = 8.67, P = .003, Cramer’s V = .29. This interaction is visually depicted in Figure 2.
FIGURE 2.
Eye movement responses in the experimental group as a function of session half and sleep state. This figure depicts the total number of trials on which EMRs were observed to the tone alone, across all learners in the experimental group who had available sleep state data. There is a session half × sleep state interaction, such that during the second half only, infants produce an EMR for the vast majority of trials during which they are in quiet sleep. Quiet sleep therefore appears to facilitate the production of conditioned EMRs
To determine if these findings were specifically related to learning and conditioned responses, we repeated the same logistic regression model but this time for the control infants. Autonomic data were available for 16 of the 17 control infants. Of the 320 possible tone alone trials, 57(17.8%) were excluded due to EMR artifact and 59 (18.4%) due to the infant being awake or missing autonomic data for that trial. This yielded a total of 224 tone-alone trials for control infants. As above, sleep state was the independent variable, EMR was the dependent variable, and half was tested as a moderator. This model yielded no main effects and no interactions, indicating that there was no association between sleep state and EMR rate in the control group.
4 | DISCUSSION
In the current study, human newborns demonstrated a robust capacity for associative learning during sleep. Sleeping full term neonates were administered a delay eyelid conditioning paradigm, in which a tone was paired with an air puff to the eye. Within a single conditioning session, 19 of 21 neonates learned the association between the tone and puff, exhibiting conditioned eye movement responses (EMRs). The infants in the experimental group more than doubled their rate of EMRs to the tone alone during the testing session, whereas a control group of infants presented with unpaired tone and puff stimuli showed consistently low rates of EMRs to the tone alone across the session. Thus, the increase in EMRs in the experimental group is a clear indication of a learned response and evidence that learning emerges quite early in the session. This study is, to our knowledge, the first to examine the role of sleep state in human infants’ likelihood of exhibiting a conditioned response. Quiet sleep appeared to facilitate the production of conditioned responses, such that learners in the experimental group were more likely to exhibit conditioned EMRs during quiet sleep compared to active sleep. This sleep state association with EMRs to the tone alone only emerged in the second half of the conditioning session, once the response had been learned. As newborns transition frequently between behavioral states, it will be important for future studies of associative learning during sleep to take state and related dynamic transitions into account.
The experimental group in the current study shows a very similar pattern of EMRs across the session as we previously reported for a different cohort (Fifer et al., 2010). The experimental groups in both studies showed a steep increase in EMRs across the session, and in both cohorts, over 90% of infants learned the conditioned response. By including a larger control group in the current study, we were able not only to confirm that the increase in EMRs in the experimental group reflected conditioning rather than arousal or other random factors, but also to compare the trajectories of EMRs across the session. While both the experimental and control groups began the session with a low baseline rate of EMRs, by the second epoch (6–12 min into the session), the experimental group already was beginning to show some evidence of learning, with a significantly higher EMR rate than the control group. Taken together, the current study and Fifer et al. (2010) suggest that the capacity for rapid associative learning during sleep is well established in most full term newborns within the first days of postnatal life. During sleep, infants must make numerous behavioral and physiological responses to escape respiratory occlusions and adapt to changes in temperature, heart rate, and blood pressure (Sahni et al., 2005), including postural adjustments and arousals (Lipsitt, 1982; Paluszynska, Harris, & Thach, 2004). The capacity for associative learning during sleep in the immediate postnatal period may promote newborn infants’ successful adaptation to the extra-uterine sleep environment.
The cerebellum is essential both to acquiring a conditioned response during delay eyelid conditioning (Cheng, Disterhoft, Power, Ellis, & Desmond, 2008; Ivkovich & Stanton, 2001; Ohyama, Nores, Murphy, & Mauk, 2003) and to producing the learned response (Clark et al., 1992). Thus, given that most full term newborns appear to learn from the delay eyelid conditioning paradigm during sleep, this method could provide a very early assessment of cerebellar function (Reeb-Sutherland & Fox, 2015), though it should be noted that it is not certain that eyelid conditioning in eyes that are already closed during sleep is mediated by the same cerebellar circuitry as traditional eyeblink conditioning. Many neurodevelopmental disorders, including Autism Spectrum Disorder, schizophrenia, dyslexia, and Attention Deficit Hyperactivity Disorder, are associated with cerebellar dysfunction and impaired eyeblink conditioning (Arndt, Stodgell, & Rodier, 2005; Coffin, Baroody, Schneider, & O’Neill, 2005; Nicolson, Daum, Schugens, Fawcett, & Schulz, 2002; Sears, Finn, & Steinmetz, 1994; Sears, Andreasen, & O’Leary, 2000), and research is needed to determine if cerebellar dysfunction in the neonatal period is a risk marker for these disorders. In developing this potential screening tool, however, it is important to consider any confounding factors that might influence performance. Quiet and active sleep states are evident in neonates (Czikk, Sweeley, Homan, Milley, & Richardson, 2002; Prechtl, 1974), and neonates transition frequently between these states (Mirmiran et al., 2003). Thus, the current study assessed sleep state during the delay eyelid conditioning paradigm and examined the role of sleep state in the production of learned responses.
The sleep characteristics we observed in our sample were typical of newborn infants, including a preponderance of active sleep (Anders et al., 1995) and frequent transitioning between behavioral states. The prevalence of active and quiet sleep remained consistent across the conditioning session; thus, the delay eyelid conditioning paradigm did not appear to alter normal neonatal sleep patterns. The likelihood of observing a conditioned EMR varied as a function of sleep state, such that in the second half of the session, EMRs in the experimental group were more likely to be observed if the infant was in quiet sleep when the tone alone trial was presented. This finding could reflect state-related differences in our ability to detect a conditioned EMR; state-related differences in the likelihood of an infant producing an EMR generally; or state-related differences in the likelihood of an infant producing a conditioned EMR specifically. One possible explanation is methodological—that it simply was more difficult to detect an EMR response against the backdrop of REM and variable autonomic activity characteristic of active sleep, as compared to quiet sleep. However, if likelihood of detection were the reason for the state-related difference observed in the second half of the session, then EMRs should also have been more prevalent during quiet sleep early in the session. This pattern was not observed in our data: In the first half of the session, as the conditioned response was being learned, EMRs during quiet sleep were rare. It was only in the second half, when a conditioned response had clearly been acquired, that the association between EMRs and quiet sleep emerged. We did not note any overt differences in amplitude or morphology of EMRs across the course of the session. Further, while EEG artifact was more common during active sleep, all trials with artifact were excluded from the current analyses. Thus, it is doubtful that likelihood of detection accounts for the greater prevalence of conditioned EMRs during quiet sleep, though future studies are needed to rule out this possibility definitively. Similarly, it does not appear to be the case that infants were more likely to produce EMRs generally during quiet sleep. Session half moderated the association of sleep state and EMRs, such that quiet sleep was only associated with EMRs in the second half of the session, once conditioning clearly was established. Also, it is important to emphasize that in the control group, for whom the tone and puff were unpaired, there was no association between sleep state and EMRs at any point in the session. This indicates that quiet sleep is specifically associated with exhibiting learned EMRs, not merely with exhibiting EMRs toward the end of the session.
Thus, overall, the current data suggests that infants were more likely to produce conditioned EMRs during quiet sleep. This is to our knowledge the first study to demonstrate that production of a conditioned response is sensitive to sleep state in human neonates. All but two infants in the experimental group were classified as learners, despite variation in sleep state, and only learners were included in the sleep state analysis. Thus, sleep state did not appear to affect the likelihood of acquiring a conditioned response. Rather, once that response had been learned, infants were more likely to exhibit it on trials during which they were in quiet sleep as compared to active sleep. Results are divergent from a study in the animal literature, in which sleeping rats acquired a conditioned response only during REM sleep, and did not produce that learned response during NREM sleep (Hennevin et al., 1995; Maho & Bloch, 1992). Given that Hennevin et al.’s study differed from the current sample in species and developmental stage, there was little basis for an a priori expectation as to which sleep state might facilitate conditioned EMRs in human neonates.
It will be important for future studies to explore the scope of this relationship between sleep state and production of a learned response. Human neonates are capable of producing several types of conditioned responses during sleep, and can learn about stimuli across several modalities. For example, neonates conditioned while awake to associate a citrus odor with tactile stimulation will turn their heads toward the citrus odor when tested during sleep (Sullivan et al., 1991). Further research is needed to determine if the observed role of sleep state in production of a conditioned response is specific to eye movement conditioning and EMRs, or generalizes to other associative learning paradigms. Future studies should also consider whether stimulus processing and motor performance may vary by sleep state, independent of conditioning. As there are studies showing clear threshold effects as a function of sleep state, such as in infant arousal thresholds (Read et al., 1998) and in olfactory processing thresholds (Murray & Campbell, 1970), it may well be that thresholds for auditory processing of the tone, for somatosensory processing of the air puff, and for emitting a motor response (eyelid movement) also differ by sleep state. Investigating such threshold effects of infant sleep state outside of a learning paradigm would then inform the interpretation of sleep state effects on conditioning.
Newborn behavioral states are fragmented, with frequent transitions between quiet sleep, active sleep, and wakefulness (Mirmiran et al., 2003). Thus, within the half hour conditioning session, many infants had several transitions between states. This variability precluded analysis in the current sample of whether quiet or active sleep facilitated learning of a conditioned response, as opposed to production of a conditioned response. This limitation could be overcome with a larger sample, allowing for the selection of subgroups of infants who spent the entirety of the first half of the session in either quiet or active sleep. Another option would be to monitor state during the session and present tone-puff pairs only when the infant was in one state, thereby allowing comparison of learning in infants who had been trained in the tone-puff association exclusively during quiet sleep to infants who had been trained only during active sleep. An additional limitation is that the current study did not quantify the precise topography, that is, amplitude, latency, and shape, of EMRs. While it would be of great interest to quantify and compare the topography of unconditioned and conditioned responses, there were two reasons this was not feasible in the current study. First, variations in head shape and face configuration relative to the electrode placement could affect the observed topography of the response in ways that would be difficult to control for statistically. Second, responses were coded from a montage of bipolar channels to facilitate determining the presence/absence of a response, but channels could and did differ from each other in the latency and amplitude of responses, making it problematic to quantify topographical characteristics.
The current study replicates and extends our prior work (Fifer et al., 2010), demonstrating that full term newborn infants reliably learn a conditioned response during sleep. Newborns learned the tone-puff association with remarkable efficiency, with their EMR patterns rapidly diverging from those of a control group. Results support the potential of the delay eyelid conditioning paradigm as a method of testing general learning capacity as well assessing cerebellar function in human newborns. Longitudinal studies are needed to determine if neonatal performance on this task could predict risk for neurodevelopmental disorders characterized by cerebellar dysfunction. As this line of research proceeds, it will be important to carefully examine and account for the role of sleep state, given current findings that quiet sleep facilitated the production of learned responses.
Acknowledgments
This research was supported by National Institute of Health grants R37 HD032774, R01MH068073, and T32 MH18264-21.
References
- Anders T, Sadeh A, Appareddy V. Normal sleep in neonates and children. In: Ferber R, Kryger MH, editors. Principles and practice of sleep medicine in the child. Philadelphia, PA: Saunders; 1995. pp. 7–18. [Google Scholar]
- Arndt T, Stodgell CJ, Rodier PM. The teratology of autism. International Journal of Developmental Neuroscience. 2005;23:189–199. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Barnes DC, Wilson DA. Sleep and olfactory cortical plasticity. Frontiers in Behavioral Neuroscience. 2014;8:134. doi: 10.3389/fnbeh.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE. How a child builds its brain: Some lessons from animal studies of neural plasticity. Preventive Medicine. 1998;27:168–171. doi: 10.1006/pmed.1998.0271. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Disterhoft JF, Power JM, Ellis DA, Desmond JE. Neural substrates underlying human delay and trace eyeblink conditioning. Proceedings of the National Academy of Sciences. 2008;105:8108–8113. doi: 10.1073/pnas.0800374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheour M, Martynova O, Naatanen R, Erkkola R, Sillanpaa M, Kero P, … Hamalainen H. Speech sounds learned by sleeping newborns. Nature. 2002;415:599–600. doi: 10.1038/415599b. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zhang AA, Lavond DG. Reversible lesions of the cerebellar interpositus nucleus during acquisition and retention of a classically conditioned behavior. Behavioral Neuroscience. 1992;106:879–888. doi: 10.1037//0735-7044.106.6.879. [DOI] [PubMed] [Google Scholar]
- Coffin J, Baroody S, Schneider K, O’Neill J. Impaired cerebellar learning in children with prenatal alcohol exposure: A comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 2005;41:389–398. doi: 10.1016/s0010-9452(08)70275-2. [DOI] [PubMed] [Google Scholar]
- Cruz S, Crego A, Ribeiro E, Goncalves O, Sampaio A. A VEP study in sleeping and awake one-month-old infants and its relation with social behavior. International Journal of Developmental Neuroscience. 2015;41:37–43. doi: 10.1016/j.ijdevneu.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Czikk MJ, Sweeley JC, Homan JH, Milley JR, Richardson BS. Cerebral leucine uptake and protein synthesis in the near-term ovine fetus: Relation to fetal behavioral state. American Journal of Physiology—Regulatory, Integrative, & Comparative Physiology. 2002;284:R200–R207. doi: 10.1152/ajpregu.00190.2002. [DOI] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers’ voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- deRegnier RA, Nelson CA, Thomas KM, Wewerka S, Georgieff MK. Neurophysiological evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. Journal of Pediatrics. 2000;137:777–784. doi: 10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- Fifer W, Byrd D, Kaku M, Eigsti I, Isler J, Grose-Fifer J, Tarullo A, Balsam P. Newborn infants learn during sleep. Proceedings of the National Academy of Sciences. 2010;107:10320–10323. doi: 10.1073/pnas.1005061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellermann LW. Chance orders of alternating stimuli in visual discrimination experiments. Journal of Genetic Psychology. 1933;42:206. [Google Scholar]
- Haddad GG, Jeng HJ, Lai TL, Mellins RB. Determination of sleep state in infants using respiratory variability. Pediatric Research. 1987;21:556–562. doi: 10.1203/00006450-198706000-00010. [DOI] [PubMed] [Google Scholar]
- Harper RM, Schechtman VL, Kluge KA. Machine classification of infant sleep state using cardiorespiratory measures. Electroencephalography and Clinical Neurophysiology. 1987;67:379–387. doi: 10.1016/0013-4694(87)90126-x. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- Hennevin E, Hars B, Maho C, Bloch V. Processing of learned information in paradoxical sleep: Relevance for memory. Behavioural Brain Research. 1995;69:125–135. doi: 10.1016/0166-4328(95)00013-j. [DOI] [PubMed] [Google Scholar]
- Herbert J, Eckerman C, Stanton ME. The ontogeny of human learning in delay, long-delay, and trace eyeblink conditioning. Behavioral Neuroscience. 2003;117:1196–1210. doi: 10.1037/0735-7044.117.6.1196. [DOI] [PubMed] [Google Scholar]
- Ivkovich D, Collins K, Eckerman C, Krasnegor N, Stanton ME. Classical delay eyeblink conditioning in 4- and 5-month-old human infants. Psychological Science. 1999;10:4–8. [Google Scholar]
- Ivkovich D, Stanton ME. Effects of early hippocampal lesions on trace, delay, and long-delay eyeblink conditioning in developing rats. Neurobiology of Learning and Memory. 2001;76:426–446. doi: 10.1006/nlme.2001.4027. [DOI] [PubMed] [Google Scholar]
- Kotilahti K, Nissilä I, Näsi T, Lipiäinen L, Noponen T, Meriläinen P, … Fellman V. Hemodynamic responses to speech and music in newborn infants. Human Brain Mapping. 2010;31:595–603. doi: 10.1002/hbm.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitt L. Infant learning. In: Quay HC, Troll L, Finlay CE, editors. Review of infant development. New York, NY: John Wiley & Sons; 1982. pp. 62–78. [Google Scholar]
- Lipsitt LP. Learning and emotion in infants. Pediatrics. 1998;102:1262–1267. [PubMed] [Google Scholar]
- Little AH, Lipsitt LP, Rovee-Collier C. Classical conditioning and retention of the infant’s eyelid response: Effects of age and interstimulus interval. Journal of Experimental Child Psychology. 1984;37:512–524. doi: 10.1016/0022-0965(84)90074-2. [DOI] [PubMed] [Google Scholar]
- Maho C, Bloch V. Responses of hippocampal cells can be conditioned during paradoxical sleep. Brain Research. 1992;581:115–122. doi: 10.1016/0006-8993(92)90350-i. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Maas YGH, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Medicine Reviews. 2003;7:321–334. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- Murray B, Campbell D. Differences between olfactory thresholds in two sleep states in the newborn infant. Psychonomic Science. 1970;18:313–314. [Google Scholar]
- Nicolson RI, Daum I, Schugens MM, Fawcett AJ, Schulz A. Eyeblink conditioning indicates cerebellar abnormality in dyslexia. Experimental Brain Research. 2002;143:42–50. doi: 10.1007/s00221-001-0969-5. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Murphy M, Mauk MD. What the cerebellum computes. Trends in Neuroscience. 2003;26:222–227. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- Paluszynska DA, Harris KA, Thach BT. Influence of sleep position experience on ability of prone-sleeping infants to escape from asphyxiating microenvironments by changing head position. Pediatrics. 2004;114:1634–1639. doi: 10.1542/peds.2004-0754. [DOI] [PubMed] [Google Scholar]
- Papousek H. Conditioned head rotation reflexes in infants in the first months of life. Acta Paediatrica. 1961;50:565–576. doi: 10.1111/j.1651-2227.1961.tb08047.x. [DOI] [PubMed] [Google Scholar]
- Prechtl HF. The behavioural states of the newborn infant (a review) Brain Research. 1974;76:185–212. doi: 10.1016/0006-8993(74)90454-5. [DOI] [PubMed] [Google Scholar]
- Read PA, Horne RS, Cranage SM, Walker AM, Walker DW, Adamson TM. Dynamic changes in arousal threshold during sleep in the human infant. Pediatric Research. 1998;43:697–703. doi: 10.1203/00006450-199805000-00020. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland B, Fox NA. Eyeblink conditioning: A non-invasive biomarker for neurodevelopmental disorders. Journal of Autism and Developmental Disorders. 2015;45:376–394. doi: 10.1007/s10803-013-1905-9. [DOI] [PubMed] [Google Scholar]
- Rovee-Collier C, Fagen JW. The retrieval of memory in early infancy. In: Lipsitt LP, editor. Advances in infancy research. Vol. 1. Norwood, NJ: Ablex; 1981. pp. 221–254. [Google Scholar]
- Rovee-Collier CK, Lipsitt LP. Learning, adaptation, and memory in the newborn. In: Stratton P, editor. Psychobiology of the human newborn. Chichester, U.K: John Wiley & Sons; 1982. pp. 147–190. [Google Scholar]
- Sahni R, Schulze KF, Kashyap S, Ohira-Kist K, Fifer WP, Myers MM. Sleeping position and electrocortical activity in low birthweight infants. Archives of Disease in Childhood. 2005;90:F311–F315. doi: 10.1136/adc.2004.055327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambeth A, Ruohio K, Alku P, Fellman V, Huotilainen M. Sleeping newborns extract prosody from continuous speech. Clinical Neurophysiology. 2008;119:332–341. doi: 10.1016/j.clinph.2007.09.144. [DOI] [PubMed] [Google Scholar]
- Sears LL, Andreasen NC, O’Leary DS. Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biological Psychiatry. 2000;48:204–209. doi: 10.1016/s0006-3223(00)00247-x. [DOI] [PubMed] [Google Scholar]
- Sears LL, Finn P, Steinmetz J. Abnormal classical eye-blink conditioning in autism. Journal of Autism and Developmental Disorders. 1994;24:737–751. doi: 10.1007/BF02172283. [DOI] [PubMed] [Google Scholar]
- Siqueland ER, Lipsitt LP. Conditioned head-turning in human newborns. Journal of Experimental Child Psychology. 1966;3:356–376. doi: 10.1016/0022-0965(66)90080-4. [DOI] [PubMed] [Google Scholar]
- So K, Adamson TM, Horne RS. The use of actigraphy for the assessment of the development of sleep/wake patterns in infants during the first 12 months of life. Journal of Sleep Research. 2007;16:181–187. doi: 10.1111/j.1365-2869.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Taborsky-Barba S, Mendoza R, Itano A, Leon M, Cotman CW, … Lott I. Olfactory classical conditioning in neonates. Pediatrics. 1991;87:511–518. [PMC free article] [PubMed] [Google Scholar]
- Tarullo A, Balsam P, Fifer W. Sleep and infant learning. Infant and Child Development. 2011;20:35–46. doi: 10.1002/icd.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Liu Y, Hou X, Sun G, Cheng Y, Luo Y. Discrimination of fearful and angry emotional voices in sleeping human neonates: A study of the mismatch brain responses. Frontiers in Behavioral Neuroscience. 2014;8:422. doi: 10.3389/fnbeh.2014.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]