Abstract
ω6- and ω3-polyunsaturated C20 fatty acids represent important components of the human diet. A more regular consumption and an accordingly sustainable source of these compounds are highly desirable. In contrast with the very high levels to which industrial fatty acids have to be enriched in plant oils for competitive use as chemical feedstocks, much lower percentages of very-long-chain polyunsaturated fatty acids (VLCPUFA) in edible plant oils would satisfy nutritional requirements. Seed-specific expression in transgenic tobacco (Nicotiana tabacum) and linseed (Linum usitatissimum) of cDNAs encoding fatty acyl-desaturases and elongases, absent from all agronomically important plants, resulted in the very high accumulation of Δ6-desaturated C18 fatty acids and up to 5% of C20 polyunsaturated fatty acids, including arachidonic and eicosapentaenoic acid. Detailed lipid analyses of developing seeds from transgenic plants were interpretated as indicating that, after desaturation on phosphatidylcholine, Δ6-desaturated products are immediately channeled to the triacylglycerols and effectively bypass the acyl-CoA pool. Thus, the lack of available Δ6-desaturated acyl-CoA substrates in the acyl-CoA pool limits the synthesis of elongated C20 fatty acids and disrupts the alternating sequence of lipid-linked desaturations and acyl-CoA dependent elongations. As well as the successful production of VLCPUFA in transgenic oilseeds and the identification of constraints on their accumulation, our results indicate alternative strategies to circumvent this bottleneck.
INTRODUCTION
Very-long-chain polyunsaturated fatty acids (VLCPUFA) have 20 or 22 carbon atoms and 4, 5, or 6 methylene-interrupted cis-double bonds in ω6- or ω3-arrangements. The nutritionally most important VLCPUFA are arachidonic (ARA; ω6-20:45,8,11,14), eicosapentaenoic (EPA; ω3-20:55,8,11,14,17), and docosahexaenoic acid (ω-3 22:64,7,10,13,16,19). Human physiology depends in various ways on these VLCPUFA, either as components of membrane phospholipids in specific tissues or as precursors for the synthesis of the different groups of eicosanoid effectors (e.g., prostaglandins) (Jump, 2002). VLCPUFA are not only required for the development of the fetal neuronal system but also contribute via a multiplicity of beneficial roles to the maintenance of health with increasing development and age, particularly by reducing the incidence of cardiovascular diseases (Demaison and Moreau, 2002). These fatty acids are either directly available as components of the diet or produced from the two essential ω6- and ω3-C18 fatty acids, linoleic (ω6-C18:29,12) and α-linolenic acid (ω3-C18:39,12,15) (Okuyama et al., 1996). Official organizations recommend that the intake of EPA should be 0.2 to 0.5 g per day (Trautwein, 2001). Thus, the daily consumption of ∼10 g (i.e., a spoonful) of a plant oil with 2 to 5% of this fatty acid would meet the recommended dietary intake. By contrast, levels of so called industrial fatty acids for use in the chemical industry have to represent ∼90% of the fatty acids in seed oils to be considered commercially viable, which is 10 to 20 times higher than the nutritionally important proportion of VLCPUFA in edible oils.
Reliable dietary sources of VLCPUFA are fish oils, whereas linoleic and linolenic acid predominate in green vegetables and some plant oils, all of which do not contain VLCPUFA. On the other hand, most processed food items with extended shelf life are characterized by high proportions of saturated fatty acids and a very high ratio of ω6/ω3-polyunsaturated C18 fatty acids, far above the recommended ratio of ∼6:1 (Trautwein, 2001). Therefore, the fatty acid profiles of modern western diets represent a rather unfavorable situation for the endogenous biosynthesis of a balanced proportion of ω6/ω3-VLCPUFA, particularly of EPA and docosahexaenoic acid (Sprecher, 2002). Because of the continuous decrease of marine resources by overfishing and the environmental impact of fish farming (Pauly et al., 2002; Hites et al., 2004), neither farmed nor caught fish can be considered a sustainable source of the VLCPUFA quantities required for a healthy nutrition of the growing human population. A solution to this predicted shortcoming may be realized by implementation of VLCPUFA biosynthesis into annual oilseed crops (Galili et al., 2002). This source would be more sustainable than production from single cell cultures of fungi or algae (Lopez Alonso and Garcia Maroto, 2000). On the other hand, these microorganisms are the most efficient primary producers of VLCPUFA and express all the enzymes required for their synthesis from C18:2 and C18:3 (Beaudoin et al., 2000; Abbadi et al., 2001; Domergue et al., 2002).
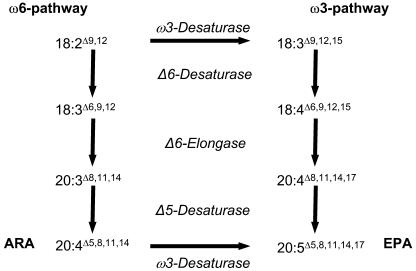
Previous experiments to change the fatty acid profiles of plant reserve triacylglycerols (TAG) by seed-specific expression of various enzymes have shown that the success of these manipulations cannot be reliably predicted, and a central question remains as to the mechanism(s) by which unusual fatty acids are accumulated in TAG (Voelker and Kinney, 2001; Drexler et al., 2003a). For a conversion of the polyunsaturated C18 fatty acids into ARA or EPA, formally three consecutive enzymatic steps are required: Δ6-desaturation, Δ6-elongation, and Δ5-desaturation (Figure 1). In a previous study, we demonstrated the reconstitution of ARA biosynthesis in yeast and showed that the front-end desaturases and elongases have strict acyl carrier specificities (Domergue et al., 2003). Here, we report experiments on the production of C20-PUFA in seeds of transgenic tobacco (Nicotiana tabacum) and linseed expressing appropriate heterologous enzymes under the control of seed-specific promoters and our effort to understand the channeling of acyl groups to TAG synthesis.
Figure 1.
Simplified ω6- and ω3-Pathways for the Biosynthesis of VLCPUFA from Linoleic (18:2) and α-Linolenic (18:3) Acid, Respectively.
RESULTS
Engineering of ARA and EPA Biosynthesis in Transgenic Plants
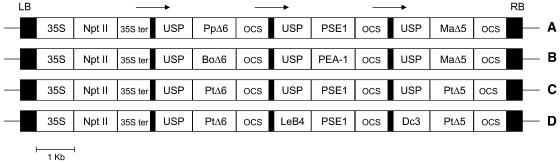
In the following, we report experiments on the production of ARA and EPA in seeds of transgenic higher plants. As heterologous coding sequences we used cDNAs encoding two regiospecifically different desaturases (Δ6 and Δ5) and a Δ6-elongase, each under the control of up to three different seed-specific promoters. The protein sequences were selected from a wide variety of VLCPUFA-producing organisms such as fungi (Mortierella alpina; Ma) (Michaelson et al., 1998), algae (Phaeodactylum tricornutum; Pt) (Domergue et al., 2002), mosses (Physcomitrella patens; Pp) (Girke et al., 1998; Zank et al., 2002), plants (Borago officinalis; Bo) (Sayanova et al., 1997), and lower animals (Caenorhabditis elegans; Ce) (Napier et al., 1998; Beaudoin et al., 2000). Four different combinations of these sequences were placed in binary vectors (Figure 2) and used for transformation of N. tabacum (high in linoleic acid, 18:2Δ9,12) and Linum usitatissimum (high in α-linolenic acid, 18:3Δ9,12,15) to identify a useful combination and a suitable expression host. For a first round of analysis, seeds of regenerated transgenic plants were collected at maturity and used for fatty acid analysis by gas–liquid chromatography (GLC)/mass spectrometry (MS).
Figure 2.
T-DNA of the Binary Vectors Used for Plant Transformation.
LB and RB, left and right T-DNA borders, respectively; USP, promoter region of the unknown seed protein of Vicia faba; LeB4, promoter of the legumin gene of V. faba; Dc3, promoter of the helianthinin gene of Daucus carota; OCS, terminator region of octopin synthase gene of A. tumefaciens; 35S, 35S promoter of Cauliflower mosaic virus (CaMV); 35S ter, CaMV 35S terminator; Npt II, neomycin phosphotransferase gene; PpΔ6, BoΔ6, and PtΔ6, Δ6-desaturases from P. patens (AJ222980), B. officinalis (U79010), and P. tricornutum (AY082393), respectively; MaΔ5 and PtΔ5, Δ5-desaturases from M. alpina (AF054824) and P. tricornutum (AY082392), respectively; PSE1 and PEA-1, Δ6-elongase from P. patens (AF428243) and C. elegans (F56H11.4), respectively.
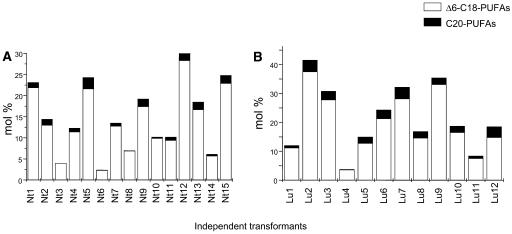
Of the four combinations tested, only the expression of constructs C and D in tobacco and linseed were successful (Figure 3). Fifty independent transgenic tobacco and 46 independent linseed lines expressing the construct C were obtained by Agrobacterium tumefaciens–mediated transformation. The fatty acid compositions of pooled T2 seeds from a selection of transgenic lines (Figure 3) showed high proportions of the two Δ6-C18-PUFAs γ-linolenic (18:3Δ6,9,12) and stearidonic acid (18:4Δ6,9,12,15) (2 to 29% in tobacco and 3 to 37% in linseed) as well as significant but disproportionately low levels of C20-PUFA (1 to 3% in tobacco and 0.5 to 8.3% in linseed; Figures 3A and 3B). These results indicate the following: (1) the promoters used enable sufficient expression in tobacco and linseed; (2) the plant enzymes (algal desaturases and moss elongase) seem to be more efficient than animal and fungal enzymes in the hosts selected; (3) to obtain significant proportions of C20-PUFA, the host plants should have high endogenous levels of linoleic and/or α-linolenic acid in their untransformed seeds.
Figure 3.
Δ6-C18- and C20-Polyunsaturated Fatty Acid Proportions in a Selection of Seeds from Primary Transformants of Tobacco and Linseed Transformed with Construct C.
Seeds were collected at 40 d after flowering (DAF) from transgenic tobacco (A) and linseed (B). FAMEs were prepared from whole seeds and analyzed by gas chromatography (GC) as indicated in Methods. Open bars, Δ6-C18-polyunsaturated fatty acid proportions; closed bars, C20-polyunsaturated fatty acid proportions.
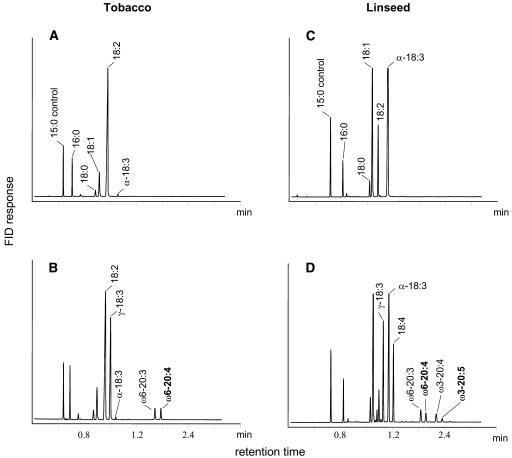
Next, groups of the most promising linseed and tobacco transformants with the construct C were subjected to DNA gel blot analysis for a selection of plants with single copy integration to facilitate comparative studies and the generation of homogeneous T3 progeny for breeding. Fatty acid analysis of transgenic T3 seed mixtures showed that in tobacco the ω6-pathway (Figure 4A) and in linseed both the ω6- and ω3-pathways (Figure 4B) were successfully reconstituted. Several new fatty acids were detected in the chromatograms of fatty acid methyl esters (FAMEs) from transgenic tobacco and linseed, respectively (Figures 4A and 4B) that are not present in the control plants. Mass spectra of their 4,4-dimethyloxazoline derivatives identified them as γ-18:3Δ6,9,12, 18:4Δ6,9,12,15, 20:3Δ8,11,14 (ω6-20:3), 20:4Δ5,8,11,14 (ω6-20:4), 20:4Δ8,11,14,17 (ω3-20:4, ARA), and 20:5Δ5,8,11,14,17 (ω3-20:5, EPA) (data not shown). The fatty acid compositions of wild-type and transgenic seeds are given in Table 1. Among the new fatty acids produced in transgenic seeds, γ-18:3Δ6,9,12 is the most abundant and accounted for ∼30 and 17% in tobacco and linseed, respectively. In linseed, 18:4Δ6,9,12,15 was the next most abundant fatty acid and accounts for ∼13%. The presence of both γ-18:3Δ6,9,12 and 18:4Δ6,9,12,15 indicates that the Δ6-desaturase from P. tricornutum used both 18:2Δ9,12 and α-18:3Δ9,12,15 as substrates in plants. This leads to a decrease in 18:2Δ9,12 in transgenic tobacco and of 18:2Δ9,12 and α-18:3Δ9,12,15 in transgenic linseed. On the other hand, the various C20-PUFA fatty acids totaled 5%. ARA accumulated up to 2% in tobacco, whereas in linseed, both ARA (up to 1.5%) and EPA (up to 1%) were found (Figure 4, Table 1). The proportions of VLCPUFA in the T3 seeds were comparable to those found in the T2 progeny, indicating that a stable phenotype was transferred to the next generation. These data demonstrate that oilseeds can in fact be transformed to produce VLCPUFA and that the proportions we observed in linseed are already close to the nutritionally relevant levels described above. For that reason, all the following experiments were conducted with linseed because of its importance as an oilseed crop.
Figure 4.
Fatty Acid Profiles of Seeds from Wild-Type and Single-Copy Tobacco and Linseed Transformants.
Seeds were collected at 40 DAF from wild-type ([A] and [C]) and transgenic ([B] and [D]) tobacco and linseed transformed with construct C (Figure 1). FAMEs were prepared from whole seeds and analyzed by GC as indicated in Methods. In this particular linseed plant, C20-polyunsaturated fatty acids accounted for 5% of the total fatty acids. It should be pointed out that none of the major peaks shown go off scale.
Table 1.
Fatty Acid Composition of Seeds from Wild-Type and Transgenic Tobacco and Linseed Plants
| Tobacco
|
Linseed
|
|||
|---|---|---|---|---|
| Fatty Acid | Wild Type | Transgenic | Wild Type | Transgenic |
| 16:0 | 9.2 ± 0.2 | 9.9 ± 0.2 | 6.2 ± 0.5 | 5.5 ± 0.5 |
| 18:0 | 2.9 ± 0.1 | 3.1 ± 0.2 | 2.7 ± 0.3 | 3.8 ± 0.2 |
| 18:1Δ9 | 11.8 ± 0.3 | 10.8 ± 0.3 | 30.8 ± 2.6 | 24.1 ± 1.7 |
| 18:2Δ9,12 | 75.4 ± 1.2 | 43.6 ± 2.6 | 11.2 ± 0.6 | 5.6 ± 1.4 |
| γ-18:3 | ND | 29.3 ± 1.7 | ND | 16.8 ± 2.8 |
| α-18:3 | 0.6 ± 0.0 | 0.7 ± 0.1 | 49.0 ± 3.1 | 30.1 ± 3.2 |
| 18:4 | ND | ND | ND | 11.4 ± 2.3 |
| ω6-20:3 | ND | 1.8 ± 0.2 | ND | 1.2 ± 0.4 |
| ω6-20:4 | ND | 1.5 ± 0.3 | ND | 1.0 ± 0.3 |
| ω3-20:4 | ND | ND | ND | 0.9 ± 0.3 |
| ω3-20:5 | ND | ND | ND | 0.8 ± 0.2 |
| ΣΔ6-C18-PUFAs | 29.3 ± 1.7 | 28.2 ± 2.5 | ||
| ΣC20-PUFAs | 3.7 ± 0.5 | 3.6 ± 1.1 | ||
Seeds from wild-type and second generation progeny of single-copy transgenic tobacco and linseed were harvested at maturity, subjected to transesterification, and analyzed by GLC as indicated in Methods. Each value is the mean ± sd of seed mixtures from five independent plants. ND, not detected.
Why Not More ARA and EPA in the Transgenic Seeds: Is the Elongase Step Limiting?
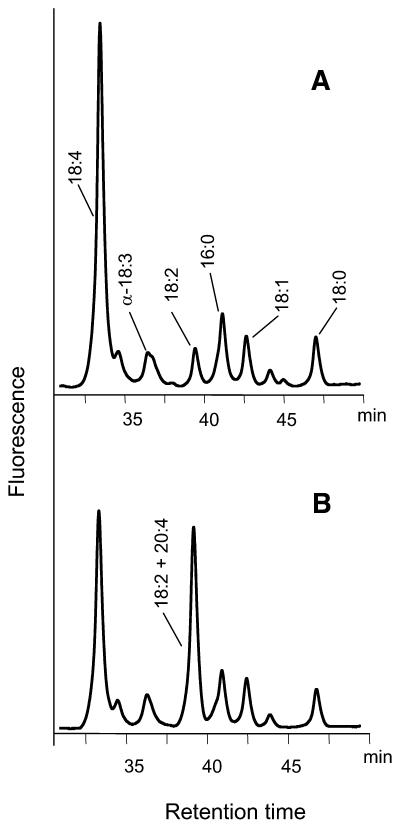
The high proportions of Δ6-desaturated C18-polyunsaturated fatty acids found in transgenic linseed (γ-18:3 and 18:4 totaling in some plants a maximum of 33%) contrasted with the low proportions of C20-PUFA, indicating that the elongating activity may limit the accumulation of C20-PUFAs. Using the same promoter in the construct C for the expression of all three genes could conceivably induce gene silencing of one of the transgenes. To see whether the low elongation can be ascribed to reduced elongase transgene transcription in seeds, transcript levels of all three heterologous genes were tested by semiquantitative RT-PCR during three different stages of seed development. RT-PCR experiments showed that the three heterologous genes (Δ6-desaturase, Δ6-elongase, and Δ5-desturase) were transcribed at similar levels (data not shown). Furthermore, a nonradioactive assay has been developed to probe the elongase activity in total extracts and microsomes of the transgenic seeds. Incubation of microsomes from the transgenic seeds with 18:4-CoA as the elongase substrate without malonyl-CoA and reducing equivalents (NADH and NADPH) showed that 18:4-CoA was recovered after extraction of total acyl-CoAs (Figure 5A). By contrast, when 18:4-CoA was incubated in the presence of malonyl-CoA and reducing equivalents with microsomes, an additional peak corresponding to 20:4-CoA (coeluting with 18:2) appeared in the chromatogram of the extracted acyl-CoAs, indicating that 18:4-CoA has been elongated efficiently to 20:4. A corresponding assay was conducted with γ-18:3-CoA. In this case, a similar extent of elongation resulted in the formation of 20:3-CoA (data not shown). According to these data, both the transcript levels and, hence, the enzyme activity of the elongase are unlikely to limit the accumulation of C20-PUFA. Therefore, we performed additional experiments to identify the cause(s) of this putative bottleneck.
Figure 5.
In Vitro Assay of Elongase Activity.
Microsomes from developing embryos were incubated with 18:4-CoA in the absence (A) and presence (B) of malonyl-CoA, NADH, and NADPH. After purification of the acyl-CoAs, they were converted to their etheno-derivatives and analyzed by HPLC. In the assay in (B), ∼50% of 18:4-CoA is elongated to 20:4-CoA.
Role of the Acyl-CoA Pool: Availability of Δ6-Desaturated Fatty Acids Is Limiting
Recent studies with elongase and desaturases expressed in yeast suggest that plant and fungal desaturases (and in particular the front-end desaturases used in this approach) use phosphatidylcholine (PC)-linked acyl groups as actual substrates, whereas the elongase uses acyl-CoAs for condensation. Therefore, the desaturation will produce Δ6-desaturated acyl groups as ester components of (phospho)lipids, whereas the Δ6-elongase requires acyl-CoA thioesters as substrates (Domergue et al., 2003). For a better understanding of this aspect of metabolic interactions in transgenic linseed, we analyzed the acyl-CoA pools of untransformed and transgenic flax seeds at different developmental stages. After extraction, the intact thioesters were converted to their fluorescent etheno-derivatives and analyzed by HPLC. In the control thioester samples (Figure 6A), palmitic, stearic, oleic, linoleic, and α-linolenic acid were present, with palmitoyl-CoA being predominant. This profile differs from the fatty acid pattern of TAG mainly by the elevated proportions of palmitic (16:0) and stearic (18:0) acid. The occurrence of linoleic and α-linolenic acid in the acyl-CoA pool confirms previous data (Stymne and Stobart, 1984) describing the acyl-CoA:lysophosphatidylcholine acyltransferase (LPCAT) activity in microsomes from developing linseeds. On the other hand, the acyl-CoA sample from transgenic tissue (Figure 6B) differed only by the presence of trace levels of stearidonic acid during all stages investigated. This indicates either a very low exchange of 18:4 between the site of desaturation in PC and the acyl-CoA pool or a rapid removal of this fatty acid from the acyl-CoA pool. The same conclusion can be drawn for γ-18:3, which coelutes as a correspondingly small proportion in the α-18:3 peak. In addition, if we assume that linoleic and α-linolenic acid residues in lipids will be desaturated equally well by the Δ6-desaturase, then both should be equally well elongated by the Δ6-elongase after transfer into the acyl-CoA pool. However, in the acyl-CoA profile, we did not detect di-homo-γ-linolenic acid (ω6-C20:3, the elongation product of γ-C18:3, eluting between 16:0 and 18:1), elongated stearidonic acid (ω3-C20:4), or any other C20-PUFAs. Addition of various C18- and C20-PUFA-CoAs to seed extracts and microsomes from the transgenic plants and subsequent extraction of total acyl-CoAs indicate that the added acyl-CoAs are recovered by extraction and are not oxidized or subjected to hydrolysis by an acyl-CoA thioesterase (Figure 5; data not shown). Therefore, the most obvious discrepancy to be explained in transgenic linseed is the following: Δ6-desaturated C18-PUFAs are found in high proportions in TAG, but they are almost completely absent from the acyl-CoA pool, whereas Δ9-unsaturated C18 fatty acids are present in roughly similar proportions in both TAG and the acyl-CoA pool. Such a distribution could be because of several mechanisms: (1) Δ6-C18-PUFAs are incorporated into TAG either via routes bypassing the acyl-CoA pool; (2) by highly efficient removal from the acyl-CoA pool giving the elongase no chance for a substantial conversion of its substrates; (3) by different subcellular compartmentation of desaturation and elongation reactions. Whatever the mechanism, the availability of Δ6-desaturated C18-CoA esters seems to prevent efficient reconstitution of VLCPUFA biosynthesis via the Δ6-elongating activity.
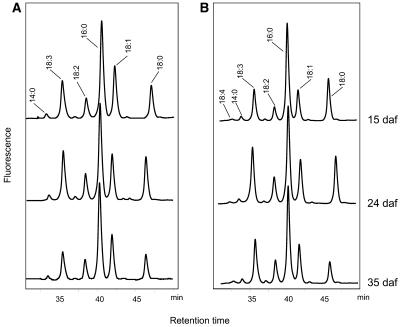
Figure 6.
Profiles of Acyl-CoAs from Developing Wild-Type and Transgenic Linseed.
Immature seeds were harvested at 14, 24, and 35 DAF from wild-type (A) and transgenic (B) linseed expressing construct C and subjected to acyl-CoA extraction. The acyl-CoAs were converted to their etheno-derivatives and analyzed by HPLC.
Channeling of VLCPUFA between Major Acyl Pools
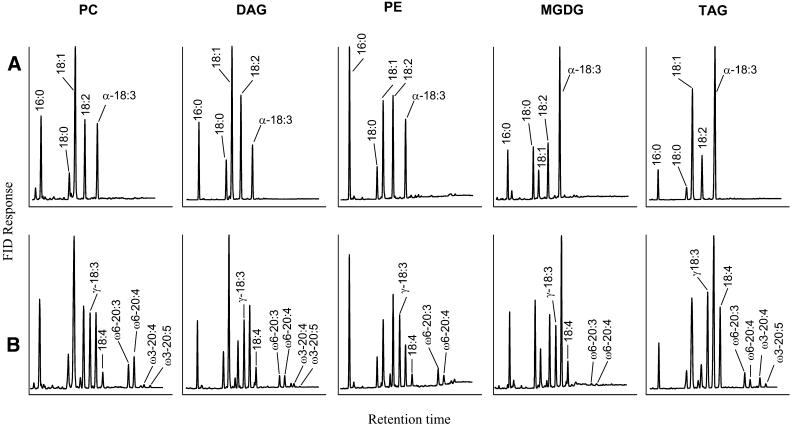
As outlined above, all Δ6-desaturated C18-fatty acids have to be generated in PC as the primary site of desaturation before delivery into the acyl-CoA pool or channeling into TAG. To prove the role of PC in Δ6-C18- and in Δ5-C20-desaturation, we next analyzed the fatty acid profile of this central metabolite and compared it with representative components of lipid groups considered as receiving fatty acids or diacylglycerol (DAG) moieties from PC. For this purpose, the developing seeds at the stage of maximal lipid deposition (24 DAF) from wild-type and T3 transgenic linseed lines were subjected to extraction of lipids followed by separation into three classes: neutral lipids, glycolipids, and phospholipids (see Supplemental Table 1 online). This fractionation scheme was chosen to ensure that no significant component escaped detection. In all three classes, the fatty acid profiles of the transgenic samples differed from those of the wild type qualitatively only by the presence of Δ6-C18-PUFA and C20-VLCPUFA, but a closer inspection reveals additional differences between the various lipid groups. γ-18:3 was nearly equally abundant in neutral lipids (15%), phospholipids (12%), and glycolipids (13%). On the other hand, 18:4 accumulated in neutral lipids (11%), whereas a reduction to equally low proportions is found in phospholipids (3.5%) and glycolipids (3%; see Supplemental Table 1 online). Furthermore, the proportion of the two ω6-C20-fatty acids derived from γ-18:3 (ω6-20:3 and ω6-20:4) was twice as high in phospholipids (total 10%) as in neutral lipids (total 5%) and five times higher than in glycolipids (2%). By contrast, the two ω3-C20-fatty acids derived from 18:4 (ω3-20:4 and ω3-20:5) were found in the neutral lipids (total 2.7%) but were hardly detected in phospholipids and glycolipids (see Supplemental Table 1 online). On the other hand, despite the fact that the ω-3 precursor α-18:3 was five times more abundant than the ω-6 precursor linoleic acid in wild-type linseed, the downstream products of the ω3-pathway were present in lower proportions than their ω-6 counterparts in the transgenic plants. This unexpected observation encouraged us to conduct a more detailed analysis of the different lipid pools.
The three lipid fractions were used to purify TAG, DAG, two individual phospholipids (PC and phosphatidylethanolamine [PE]), and the two galactolipids monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (data not shown) because plastidial glycolipids of eukaryotic origin represent another pool of DAG derived from microsomal PC. Before considering the differences in fatty acid methyl esters between wild-type and transgenic samples (Figure 7), it is useful to compare in wild-type seeds the fatty acid profiles of PC with the two major lipid pools receiving fatty acids from PC:MGDG and TAG (Figure 7). It is obvious that α-18:3 is the predominant component in the accepting lipids MGDG and TAG, whereas the PC profile is dominated by 18:1, which originates from the plastid-derived acyl-CoA pool (Figure 7A). The lipids from transgenic seeds differ by the presence of Δ6-C18-PUFA and C20-PUFA but reflecting to some extent the observations made with wild-type lipids. Figure 7B shows that 18:4 and ω3-C20-PUFAs were concentrated in TAG contrasting with their low proportions in PC, DAG, PE, and MGDG. γ-18:3 was found nearly evenly distributed in all lipids. The major C20-PUFAs were ω6-C20-PUFAs 20:3-ω6 and 20:4-ω6 (4.2 and 5.3%, respectively), which were present in much greater proportions than the two ω3-C20-PUFAs and which were enriched in PC and DAG. γ-18:3 and 18:4 in MGDG (Figure 7B) must be considered as being of eukaryotic (i.e., microsomal) origin. In addition, very low proportions of ω6-C20-PUFAs were also present. These results indicate that major fluxes of VLCPUFA to glycolipids may occur via DAG. However, it is noteworthy that 18:4 and its derived products are concentrated in TAG (Figure 7B) but not in PC, DAG, PE, and MGDG. This in turn indicates that, in addition to the contribution of the DAG pool derived from PC, these fatty acids might be channeled by other enzymatic mechanisms. From Figure 7 it is also evident that the proportion of 16:0 in TAG is rather low compared with PC and the acyl-CoA pool, which both feed TAG synthesis.
Figure 7.
Fatty Acid Profiles of PC, DAG, PE, MGDG, and TAG from Developing Wild-Type and Transgenic Linseed Seeds (24 DAF).
PC, DAG, PE, MGDG, and TAGs were purified by high-performance thin-layer chromatography (HPTLC) from the respective lipid fractions as indicated in Methods and directly subjected to transesterification for GC analysis.
(A) Wild-type seeds.
(B) Transgenic seeds.
Positional Distribution and Pairing of Fatty Acids
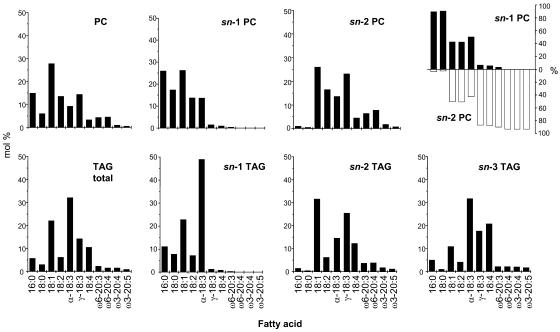
To better understand the acyl group channeling between PC, acyl-CoAs, and TAG, we performed a positional analysis of fatty acids in PC and TAG. In the latter case, we used a method allowing the differentiation of all three positions (sn-1, sn-2, and sn-3) because their acyl profiles may point to the enzymatic reactions contributing most to filling up a particular position. Furthermore, the analysis of PC was extended to a study of the molecular species to find out whether all or only specific pools of PC participate in these metabolic conversions. Figure 8 shows the positional distribution of fatty acids from PC and TAG of seeds from transgenic linseed (24 DAF). The acyl groups of PC can be separated into three groups (Figure 8, top row, right): sn-1–confined saturated fatty acids (16:0 and 18:0); acyl groups equally distributed between the sn-1 and sn-2 positions (18:1, 18:2, and α-18:3); acyl groups concentrated in the sn-2 position (all carrying a Δ6-double bond as well as their elongated derivatives). This distribution supports the sn-2 regiospecificity of the Δ6- and Δ5-desaturase (Domergue et al., 2003) and suggests that the products after elongation are returned with high preference into the same position. More details will be discussed below in comparison with the TAG positions.
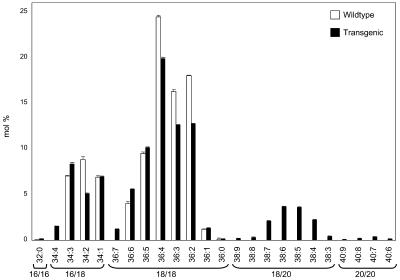
Figure 8.
Regiospecific Distribution of Fatty Acids in PC and TAG from Developing Seeds of Transgenic Linseed (24 DAF).
The positional analysis of fatty acids from PC and TAG were determined as indicated in Methods. In all plots, except for the right PC plot, mol % refers to the percentage of each acyl group in the lipid class or at a particular position in that lipid class. In the right PC plot, “%” refers to the percentage of each fatty acid found in the sn-1 or sn-2 position. Each value is the mean from three independent experiments.
To find out whether the Δ6-desaturated and elongated acyl groups are combined with preferred acyl groups in the sn-1 position, we analyzed the fatty acid combinations occurring in PC from wild-type and transgenic seeds. As seen in Figure 9, wild-type PC is made up of 16/18 and 18/18 combinations. Together with the positional analysis (Figure 9), the 16/18 combinations are fully identified with regard to regiospecificity. A similar assignment is not possible with 18/18 combinations because the MS technique used can only identify the most predominant regioisomers present in mixtures with isobaric and regiospecific isomers. Therefore, only 36:6 (18:3/18:3), 36:2 (18:1/18:1), and 36:1 (18:0/18:1) are fully assigned, whereas all other combinations contain several species. The predominant combinations with the two constituent fatty acids in roughly similar proportions in both sn-positions are 18:3/18:2 (36:5), 18:3/18:1 (36:4), and 18:2/18:1 (36:3). A detailed analysis is provided in the supplemental data (see Supplemental Table 2 online). From these patterns, we conclude that the normal Δ12- and Δ15-desaturases as well as the LPCAT accept both 16/18 and 18/18 combinations. In PC from the transgenic seeds, additional 18/20 and 20/20 combinations and the new 16:0/18:4 (34:4) and 18:3/18:4 (36:7) occur. The proportions of the new 18/20 combinations show that most of the Δ6-desaturated and subsequently elongated acyl groups are combined with a C18-PUFA and not with C16:0. Nevertheless, 16:0/20 combinations can be detected in very low proportions in the corresponding 36:x combinations. From these data, we conclude that the large majority of the elongated Δ6-desaturated acyl groups are combined with an unsaturated C18-fatty acid. Unfortunately, we cannot extend this conclusion to γ-18:3 itself because the MS technique used does not differentiate between α- and γ-18:3.
Figure 9.
Molecular Species of PC from Developing Wild-Type and Transgenic Linseed (24 DAF).
Finally, we performed a stereospecific analysis of TAG from wild-type (see Supplemental Figure 1 online) and transgenic linseed, but only the data from the transformants are included in Figure 8 for an immediate comparison with the profiles in equivalent PC positions. Our analysis of the wild-type TAG supports previous data (Mattson and Volpenhein, 1963) and shows the exclusion of saturated fatty acids from the sn-2 position but a more or less random distribution of 18:1 and 18:3 over all three positions, whereas 18:2 is enriched at the sn-2 position. By contrast, the three sn-positions of the transgenic TAG show different profiles. Confining the positional analysis to a lipase hydrolysis would not differentiate between sn-1 and sn-3, emphasizing the need for the more complicated analysis described here. The sn-1 profile is dominated by α-18:3, whereas 16:0 and 18:0 are significantly reduced when compared with the sn-1 position of PC. Δ6-Desaturated fatty acids and their derivatives are nearly absent from this position paralleling the corresponding PC pattern. In the sn-2 profile, 16:0 and 18:0 are nearly absent, similar to the sn-2 position of PC. This similarity extends to the presence of γ-18:3 and 18:4 as well as to the C20 series. The proportion of γ-18:3 is highest in this position, but the two C20-ω6-members (in contrast with the ω3-members) are reduced compared with the PC pattern. Nevertheless, the pairs of sn-1 and sn-2 profiles of PC and TAG display some basic similarity. By contrast (and as might be expected from the complicated biochemical interactions to be discussed below), the acyl mixture at the sn-3 position of TAG differs most from all the others. It contains significantly less 16:0 and 18:0 than the sn-1 positions and also less 18:1 than any other TAG positions and the acyl donor pools (acyl-CoA thioesters, free DAG, PC-bound DAG, and its different sn-positions). The sn-3 profile is dominated by α-18:3 followed by significant and approximately equal proportions of 18:4 and γ-18:3. The four different C20 fatty acids are present in roughly equal proportions similar to the sn-2 profile but significantly different from the sn-2 position of PC with the characteristically uneven proportions of the C20-ω6- and C20-ω3-pairs.
Some of the important results of this stereospecific TAG analysis are the following: (1) all the new (i.e., non-native) C18 and C20 fatty acids are not present at the sn-1 position, (2) γ-18:3 is most abundant at the sn-2 position, and (3) by contrast, 18:4 is highest in the sn-3 position.
Taken together, these data show that the various pools (acyl-CoA thioesters, backbones, and the different sn-positions of PC, free DAG, galactolipids, and TAG) display fatty acid profiles with significant differences, in particular regarding the proportions of the heterologously expressed new fatty acids. This suggests the operation of selectivity and preference in the exchange of single acyl groups and/or complete DAG moieties between these pools. Thus, ω3-C18- and ω3-C20-PUFA are depleted in PC and efficiently moved to TAG. Compared with this, ω6-18:3 and in particular its ω6-C20-PUFA metabolites, appear to be effectively retained in the PC pool. In addition, a considerable proportion of 18:1, 18:2, and α-18:3 is moved through the acyl-CoA pool, whereas Δ6-desaturated C18-PUFA and their elongation products can hardly be detected in this pool. The significance of these observations will be discussed in the next section.
DISCUSSION
The goal of our study was to produce transgenic plant oils enriched with VLCPUFA. The data presented demonstrate a successful reconstitution of C20-VLCPUFA biosynthesis in oil-synthesizing seeds of higher plants. The genetic basis of this implementation in linseed was the heterologous expression of three genes encoding a Δ6-desaturase, a Δ6-elongase, and a Δ5-desaturase, the primary enzymes of VLCPUFA biosynthesis. Of particular significance is the fact that, according to the dietary values recommended by nutritional organizations, the proportions of ∼5% C20-PUFA reached in our transgenic seed oils would already be sufficient to satisfy these requirements. However, for expanded agricultural use, the current reported levels of ARA and EPA may still require some enhancement. On the other hand and perhaps of equal or greater significance, our data now offer new insights into the metabolic fluxes governing the production and deposition of these new fatty acids in seeds.
In previous experiments with the enzymes used in this study, we demonstrated that the Δ6- and Δ5-desaturation take place at the sn-2 position of PC and that the Δ6-elongase uses acyl-CoAs as substrates (Domergue et al., 2003). In addition, when expressed in yeast and supplemented with an exogenous fatty acid mixture reflecting that present in linseed, the moss Δ6-elongase very efficiently elongated γ-C18:3 and 18:4, converting >50% of these substrates to C20-PUFAs (Zank et al., 2002). However, despite the accumulation of high proportions of Δ6-desaturated C18-fatty acids in this study, our transgenic linseed lines did not use these substrates efficiently for elongation; therefore, the heterologous biosynthesis of VLCPUFA did not proceed significantly beyond the first desaturation step. Analysis of the acyl-CoA pool of the transgenic seeds at different developmental stages showed that γ18:3- and 18:4-CoAs and their elongation products were hardly detectable, whereas 18:2- and α-18:3-CoA were present. This can be interpreted in two ways: either there was a selectively slow exchange of Δ6-desaturated acyl groups between PC and the acyl-CoA pool, or the Δ6-desaturated acyl groups were removed very rapidly from this pool by enzymes having higher affinities for these substrates than the elongase and simultaneously discriminating against 18:1, 18:2, and α-18:3. On the other hand, RT-PCR and the results presented in Figure 5 indicate that the elongase was transcribed correctly during seed development, resulting in high enzymatic activity during the main phase of lipid accumulation. Therefore, we assume that substrate availability rather than catalytic activity limited the elongation. This in turn suggests that additional mechanisms may contribute to the channeling of most of the Δ6-C18-PUFA from PC to TAG by enzymes not dependent on the presence of these PUFA in the acyl-CoA pool. Our additional experiments were directed to understand these metabolic fluxes and to find out which of various alternatives contributed most to the acyl channeling in TAG synthesis.
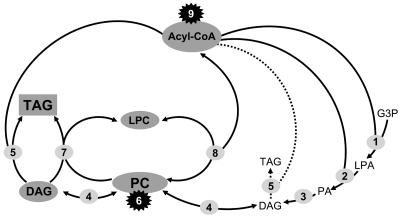
Current understanding suggests that several reactions contribute to the flow of acyl groups between the various pools relevant in TAG biosynthesis, and some of them are in fact acyl-CoA independent (Figure 10). Several different mechanisms may be involved in the synthesis of DAG and TAG. DAG may be released from phosphatidic acid after de novo synthesis, but DAG may also be released from PC in the reverse cholinephosphotransferase (CPT) reaction. On the other hand, PC synthesis itself requires acyl-CoA–dependent de novo synthesis of DAG via phosphatidic acid. In addition, DAG may also result from an acyl-CoA-dependent acylation of monoacylglycerol (Beaudoin and Napier, 2004). The final conversion of DAG to TAG may be catalyzed by the acyl-CoA:DAG acyltransferase (DAGAT) (Voelker and Kinney, 2001) and by two acyl-CoA–independent reactions, for example, those catalyzed by the DAG:DAG transacylase (DDAT; not included in Figure 10) (Stobart et al., 1997) and the phospholipid:DAG acyltransferase (PDAT). This enzyme transfers the acyl-chain from the sn-2 position of PC to DAG yielding TAG and sn-2-lyso-PC (Dahlqvist et al., 2000). The acyl-CoA pool is fed mainly by acyl group export from plastids (16:0, 18:0, and 18:1) but changed in composition by the reverse reaction of the LPCAT (Stymne et al., 1983; Stymne and Stobart, 1984) by elongases and acyl-CoA synthases. These activities result in a dynamic and heterogenous acyl-CoA pool that is subject to further modification because of more or less selective removal by acyltransferases for de novo synthesis of phosphatidic acid and the deposition of acyl groups in TAG (Beaudoin and Napier, 2004). Thus, there are several acyl-CoA–independent routes by which PC-bound Δ6- and Δ5-desaturated acyl groups could be incorporated into TAG, explaining their absence from the acyl-CoA pool. The enzymes responsible for this channeling are CPT (Δ6-desaturated acyl groups at least in the sn-2 position of TAG), DDAT (Δ6-desaturated acyl groups at least in the sn-2 and sn-3 positions of TAG), and PDAT (Δ6-desaturated acyl groups in addition in the sn-3 position of TAG).
Figure 10.
Accumulation of Linseed TAG by Channeling of Acyl Groups through PC.
The stepwise acylation of glycerol-3-phosphate (G3P) by acyl-CoA:1-glycerol-3-phosphate acyltransferase (G3PAT; 1) and acyl-CoA:1-acyl-glycerol-3-phosphate acyltransferase (2) yields 1-acyl-glycerol-3-phosphate (LPA) and phosphatidic acid (PA), which is hydrolyzed by phosphatidic acid phosphatase (3) to give DAG. Its conversion to TAG by acyl-CoA:diacylglycerol acyltransferase (5) immediately after the primary formation of DAG by the Kennedy pathway represents a minor alternative for TAG synthesis in linseed (indicated by the dotted arrow). The major part of DAG is converted to PC in a reversible reaction catalyzed by CDP-choline:diacylglycerol cholinephosphotransferase (CPT; 4). PC is the substrate for acyl group desaturases (6). They may accept both the sn-1 and sn-2–localized acyl groups (the genuine enzymes), or their action may be confined to the sn-2–bound acyl chains (the heterologously expressed Δ6- and Δ5-desaturase). In a reversible reaction, the acyl-CoA:lyso-phosphatidylcholine acyltransferase (8) equilibrates the sn-2–bound acyl group with the acyl-CoA pool. The formation of C20-polyunsaturated fatty acids by the heterologously expressed acyl-CoA elongase (9) occurs by elongation of Δ6-C18–polyunsaturated acyl-CoA and thus depends on their release from PC into the acyl-CoA pool by the LPCAT (8). The activities of the desaturases (6) and elongase (9) together with the reversibility of LPCAT (8) and CPT (4) result in a continuous change of the acyl groups of the acyl-CoA pool, PC, and DAG, which all participate in this equilibration. Only after passage through PC, a highly desaturated DAG is finally converted into TAG. This channeling is catalyzed by two enzymes, the DAGAT (5) mentioned before and the phospholipid:diacylglycerol acyltransferase (PDAT; 7) that transfers the sn-2–bound acyl group from PC to the sn-3 position of TAG. These major routes, deduced by labeling studies with wild-type linseed (Slack et al., 1983; Stymne and Stobart, 1984), are supported by the acyl profiles of the various pools (acyl-CoA, PC, DAG, and TAG) studied in this investigation with transgenic linseed.
Earlier studies (Slack et al., 1978, 1983; Somerville and Browse, 1991) suggested that in linseed the DAG and PC pools are essentially kept in equilibrium by the action of CPT. The major flux of glycerol and fatty acids incorporated into TAG proceeds predominantly via PC rather than depending on the stepwise use of 18:2- and 18:3-CoAs (derived from PC by exchange) for the uninterrupted and sequential formation of phosphatidic acid, DAG, and TAG via the classical Kennedy pathway. Taking into account that the Δ6-desaturase is specific for the sn-2 position of PC and that Δ6-C18-PUFA may not be efficiently exchanged from the sn-2 position of PC by the LPCAT, the DAG pool becomes enriched with the sn-2–bound Δ6-C18-PUFA by conversion of PC to DAG by CPT. The fact that the fatty acid profile of total DAG resembled that of PC supports this assumption. These DAG molecules can then be incorporated into TAG by the action of DAGAT, PDAT, and DDAT or serve as substrates for the synthesis of other phospholipids (PE) and of glycolipids in the plastids (Figure 10).
On the other hand, the low level of 18:4 in PC and DAG and its abundance in TAG suggests that after desaturation this fatty acid is immediately shuttled to TAG. A possible mechanism for this channeling could be a high activity of the PDAT enzyme in addition to the channeling via the CPT-catalyzed release of DAG backbones. Positional analyses of PC and TAG from developing seeds indicate that γ-18:3 and 18:4 were absent from the sn-1 positions of both lipids. γ-18:3 was confined to the sn-2 position in PC and found at the sn-2 and sn-3 positions of TAG with some preference for the sn-2 position. By contrast, 18:4 was found to accumulate preferentially at the sn-3 position of TAG (Figure 8). The enrichment of 18:4 at the sn-3 position of TAG can be explained in two ways. One possibility is that the DAGAT prefers 18:4-CoA as substrate to form TAG rich in this fatty acid. This could explain the observation that in developing transgenic seeds 18:4-CoA is hardly detected in the acyl-CoA pool. An alternative possibility is that a PDAT-like enzyme is responsible for the direct channeling of 18:4 from the sn-2 position of PC to the sn-3 position of TAG. This alternative would be in line with the fact that 18:4 is a major component in the sn-3 position of TAG, but only a minor component in PC, absent in the acyl-CoA pool and only marginally elongated.
At this point it is interesting to compare our data on the positional distribution of Δ6-desaturated fatty acids in transgenic linseed with those determined previously in TAG from two transgenic Brassica species and the well known γ-18:3–rich oils (Gunstone, 1992) from evening primrose (Oenothera biennis), black currant (Ribes nigrum), and borage (B. officinalis). The last three plants direct γ-18:3 into the sn-2 and sn-3 positions with only very minor proportions in sn-1. The Δ6-desaturases of these plants also seem to be specific for the sn-2 position of PC (Stymne and Stobart, 1986; Domergue et al., 2003). In transgenic Brassica juncea expressing a Δ6-desaturase from Pythium irregulare, γ-18:3 produced in the seeds was not found in polar lipids, but almost all of it was targeted preferentially to the sn-2 position of TAG, whereas 18:4 was equally present in sn-2 and combined (sn-1 + sn-3) positions (Hong et al., 2002). In the related species B. napus, coexpression of Δ12- and Δ6-desaturases from M. alpina resulted also in a high accumulation of γ-18:3 in TAG. But in this case three-quarters of this fatty acid were located at sn-1 + sn-3 positions (not differentiated) of the TAG (Liu et al., 2001). These unexpected differences demonstrate that even among closely related plant species different mechanisms do occur for the channeling of Δ6-desaturated acyl groups into TAG.
A further complication results from the fact that TAG synthesis occurs in parallel to phospholipid synthesis in the endoplasmic reticulum rather than in a separate compartment such as the differentiated oil body itself (Beaudoin and Napier, 2004). Therefore, the confinement of specifically desaturated fatty acids to TAG has to be ascribed to the specificity of the enzymes mentioned above, which in addition may prefer (or be associated with) pools of specific molecular species. Whether this could be because of a lateral heterogeneity of endoplasmic reticulum membranes is still an open question (Vogel and Browse, 1996). Our analysis of the molecular species of PC from wild-type and transgenic seeds (24 DAF) indicates that the C20 fatty acids in the transgenic samples are found to be primarily combined with C18 fatty acids rather than with 16:0. These species seem to be precursors for the enrichment of C20 fatty acids in TAG. Therefore, the different pools of PC may not contribute to the same extent to TAG synthesis and its enrichment with polyunsaturated fatty acids. This has been suggested in previous studies dealing with plants accumulating polyunsaturated fatty acids (Roughan and Slack, 1982; Beaudoin and Napier, 2004).
Our data with linseed indicate that after desaturation, Δ6-C18-PUFA are likely to be channeled from PC directly to other lipids, preventing their exchange with the acyl-CoA pool and efficient subsequent elongation. Accordingly, the introduction of lipid-linked Δ6-desaturation products into the acyl-CoA pool represents the most severe bottleneck for the production of VLCPUFA in transgenic linseed. A possible candidate enzyme regulating the above mentioned bottleneck (i.e., making available the Δ6-desaturated C18-PUFA for elongation) may be LPCAT (Stymne and Stobart, 1984). In linseed cotyledons, this enzyme was shown to discriminate against saturated fatty acids, whereas 18:2 and α-18:3 produced in PC were rapidly returned to the acyl-CoA pool (Stymne and Stobart, 1984). Our new data confirm this observation and can be interpreted as showing that the linseed LPCAT enzyme may discriminate in addition against non-native Δ6-polyunsaturated fatty acids, in particular for the intrinsically slower reverse (PC to acyl-CoA) reaction (Stymne et al., 1983). At present, we do not know whether the same activity in different oilseed crops displays a similar selectivity. However, we would expect a significant increase in Δ6-elongation by the coexpression in linseed of a LPCAT activity accepting Δ6-desaturated C18 fatty acids.
Strategies to Improve VLCPUFA Synthesis in Transgenic Plants
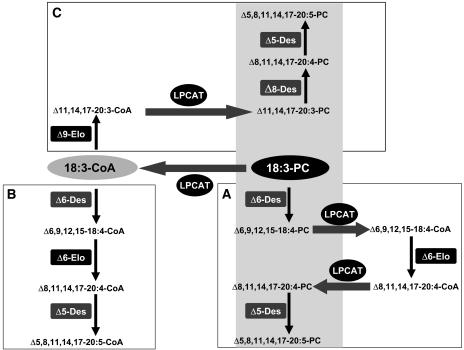
The identification of the bottleneck in our approach raises additional interest in alternative strategies for VLCPUFA biosynthesis, as briefly outlined here (Figure 11). For example, the alternative shown in Figure 11B makes use of animal Δ6- and Δ5-front end desaturases that have been shown to use acyl-CoA as substrates (Okayasu et al., 1981; Irazu et al., 1993). With such enzymes, the conversion of linoleic or α-linolenic acid to ARA or EPA would be accomplished exclusively on the acyl-CoA track and thus avoid the switching between lipids and acyl-CoA (Figure 11B). Analyses of acyl-CoA profiles in developing seeds from linseed (our data) and rapeseed (Larson et al., 2002) have shown that this pool is indeed nearly in equilibrium with regard to the primarily lipid-linked Δ9-polyunsaturated desaturation products, such as linoleoyl and α-linolenoyl residues.
Figure 11.
Differences in the Interplay between Desaturases, Elongases, and Acyltransferases in the Biosynthesis of VLCPUFA in Transgenic Plants.
Three different strategies can be used to generate VLCPUFA.
(A) The lipid-linked desaturation pathway was followed by our approach and requires both forward and reverse reactions of a Δ6-specific LPCAT, which is the limiting step in linseed.
(B) The acyl-CoA pathway, where both the elongation and desaturation occur in the acyl-CoA pool, requires acyl-CoA front-end desaturases so far only known from mammals. PC, phosphatidylcholine; Elo, elongase; Des, desaturase; light gray shading represents the membrane environment.
(C) An alternative pathway relying on the initiating Δ9-elongation step in the acyl-CoA pool followed by lipid-linked desaturations has been verified in leaves (Qi et al., 2004).
The abundance of these Δ9-polyunsaturated C18 fatty acids in plant acyl-CoA pools suggests that the bottleneck observed in the lipid-linked Δ6-desaturation/Δ6-elongation sequence (Figure 11A) could be circumvented by yet another alternative depicted in Figure 11C. It depends on the sequential action of a Δ9-elongase and a Δ8-desaturase. cDNA sequences for both enzymes have been cloned from algae (Wallis and Browse, 1999; Qi et al., 2002). On expression in developing seeds this Δ9-elongase would have access to the pool of Δ9-C18–polyunsaturated acyl-CoAs and produce Δ11-C20–polyunsaturated acyl-CoAs, which after a presumed incorporation into PC would become substrates for the subsequent desaturation by the Δ8-desaturase forming lipid-linked Δ8-C20-PUFA. They in turn would serve immediately as substrate for a lipid-linked Δ5-desaturase. This Δ9-elongation/Δ8-desaturation sequence requires an additional acyltransferase (LPCAT) for incorporation of the Δ11-C20-PUFA into phospholipids to make them acceptable by the Δ8-desaturase. This forward acylation of lyso-phospholipids may be less selective than the reverse reaction required for releasing lipid-bound acyl groups into the acyl-CoA pool. Recently, a successful implementation of this strategy in nonseed tissues of Arabidopsis thaliana led indeed to the accumulation of C20-PUFAs in leaves (Qi et al., 2004). Whatever strategy used, the selectivity of a LPCAT seems to play a critical role in the successful reconstitution of the conventional C20-PUFA biosynthetic pathway.
Further efforts will be directed toward increasing the levels of C20-PUFAs reported in this study. However, our data clearly demonstrate the feasibility of the production of these nutritionally important fatty acids in transgenic oilseeds. A supporting approach might be to produce similar levels of these beneficial fatty acids in green vegetables, which are particularly rich in the precursor α-18:3 (Qi et al., 2004). The availability of such oils and vegetables enriched in VLCPUFA will be a new addition to the list of functional food items of plant origin designed for increased levels of minor, but nutritionally highly relevant, components such as β-carotenes (Ye et al., 2000), tocopherols (Shintani and DellaPenna, 1998), and vitamin C (Agius et al., 2003).
METHODS
Materials
Restriction enzymes, polymerases, and DNA modifying enzymes were obtained from New England Biolabs (Frankfurt, Germany). Linum usitatissimum (cv Flanders) was obtained from CEBECO (Vlijmen, The Netherlands). All components for plant media were obtained from Duchefa (Haarlem, The Netherlands). The Agrobacterium tumefaciens strain C58C1 ATHV, a derivative of strain EHA 101 (Hood et al., 1986), was kindly provided by J. Dettendorfer (KWS, Einbeck, Germany). All other chemicals were from Sigma (St. Louis, MO) unless indicated otherwise.
Plant Transformation Constructs
The binary vectors pCAMBIA2300 (CAMBIA, Canberra, Australia) and pGPTV (AJ251014), which both use the nptII gene with the CaMV 35S promoter as the selectable marker, were used for the construction of plasmids for plant transformation. For the generation of constructs A, B, and C (Figure 2), a triple cassette containing the USP promoter (Bäumlein et al., 1991), the OCS terminator, and three different polylinkers between each promoter and terminator were first inserted into the pUC19 vector (Pharmacia, Erlangen, Germany), yielding the USP123OCS plasmid. The open reading frames of the different desaturases and elongases were modified by PCR to create appropriate restriction sites adjacent to the start and stop codons, cloned into the pGEMT-T vector (Promega, Madison, WI), and sequenced to confirm their identity. The open reading frames were then released using the restriction sites created by PCR and successively inserted into the same sites of the polylinkers of the USP123OCS plasmid. The resulting cassette, containing the three genes each under the control of the USP promoter, was released by digesting the USP123OCS plasmid with AscI or EcoRI and HindIII and cloned into the corresponding sites of the binary vectors pGPTV and pCAMBIA2300, respectively. For the generation of construct D (Figure 2), the USP promoter at the second and third positions of the cassette in the USP123OCS plasmid was replaced by the LeB4 promoter (Fiedler and Conrad, 1995) and the Dc3 promoter (Thomas, 1993), respectively. The corresponding plant construct was then generated as described above. Plasmid DNA from all constructs was isolated and purified according to standard methods. All vectors were transferred into the Agrobacterium strain C58C1 ATHV via electroporation.
Plant Transformation
Linseed transformation was performed according to Drexler et al. (2003b). Regenerated shoots were selected on MS medium with 1.5% (w/v) sucrose, 400 mg/L of carbenicillin, and 60 mg/L of G418. After rooting they were transferred to pots and grown at 22°C under 16-h daylight in a growth chamber. The flowers were labeled at anthesis, and immature and mature seeds were harvested at 24 and 40 d after flowering (DAF), respectively. The tobacco (Nicotiana tabacum) transformation was conducted as described previously (Jongsma et al., 1995).
Fatty Acid Analysis
Ten milligrams of seeds from wild-type and transgenic plants were used for preparation and analysis of fatty acid methyl esters as described before (Domergue et al., 2002). For the analysis of immature seeds, seed probes were used to prepare parallel samples of cell debris, the wash fraction, and the acyl-CoA extract (see below), which were subjected to acidic transesterification and analyzed by GLC as described (Domergue et al., 2002). FAMEs were identified by comparison with appropriate reference substances and by GLC/MS of their 4,4-dimethyloxazoline derivatives as described previously (Sperling et al., 2000).
Preparation of Cell-Free Homogenates and Microsomes
Transgenic embryos (20 to 24 DAF, 100 to 200 mg fresh weight) were isolated at 0°C and homogenized in grinding medium (100 mM Hepes buffer, 0.32 M sucrose, 0.1% BSA, and 10 mM 2-mercaptoethanol, pH 7.2). After filtration through Miracloth (Merck, Darmstadt, Germany), protein was estimated according to Bradford (1976), and an aliquot of the cell-free homogenate was directly used for assaying elongation activity. Microsomes were prepared as indicated by Dahlqvist et al. (2000).
Assay of Elongation Activity
An aliquot of the cell-free extract (200 μL, 200 μg protein) or microsomes (500 μL, 150 μg protein) was incubated at 30°C for 1 h with 90 mM Hepes, pH 7.2, 10 mg/mL of BSA, 1 mM ATP, 0.5 mM NADH, 0.5 mM NADPH, 2 mM MgCl2, 100 μM malonyl-CoA, and 23.2 μM 18:4-CoA in a final volume of 1 mL. The reaction was stopped by adding 200 μL of 3 M acetic acid and 25 μL of a saturated (NH4)2SO4 solution. The mixture was briefly centrifuged, and the acyl-CoA fraction was purified on a Sep-pak column (Strata C18-E; Phenomenex, Torrance, CA) using acetonitrile as the eluting solvent. The samples were dried under argon and derivatized as indicated below.
Acyl-CoA Analysis
Saturated and monounsaturated acyl-CoA esters with acyl chain lengths from C12 to C18 were obtained from Sigma. Polyunsaturated acyl-CoAs (18:2-, α18:3-, γ18:3-, 18:4-, 20:2-, 20:3ω6-, 20:3ω3-, 20:4ω6-, and 20:4ω3-CoAs) were synthesized enzymatically using an acyl-CoA synthetase from Pseudomanas sp (Sigma). The reaction mixture (200 μL) containing 100 mM Tris-HCl, pH 8.1, 10 mM MgCl2, 5 mM ATP, 5 mM CoASH, 2 mM DTT, 25 μM free fatty acid, and 2.5 units of acyl-CoA synthetase was incubated at 37°C for 2 h. The reaction was stopped with 50 μL of glacial acetic acid/ethanol 1:1 (v/v), and the samples were washed with petroleum ether to extract residual free fatty acids. After purification of the acyl-CoAs on a Sep-pak column (Strata C18-E; Phenomenex) using acetonitrile as the eluting solvent, the samples were dried under argon and dissolved in 50 mM Mes, pH 5.0.
Acyl-CoA analysis was performed as described by Larson and Graham (2001) with slight modifications: acyl-CoAs were extracted from 20 mg of immature seeds (24 DAF) and washed with petroleum ether. The resulting extract was dried under argon at 50°C. Chloracetaldehyde reagent (300 μL) was then added to the samples, and the derivatization of acyl-CoAs to their etheno-derivatives was conducted at 85°C for 20 min.
HPLC analysis was performed with Thermoquest HPLC equipment (Thermoquest, Egelsbach, Germany) using a LUNA 150 × 2.0 column with phenyl-hexyl coated 5-μm silica particles (Phenomenex) under the same conditions as described by Larson and Graham (2001).
Lipid Analysis
Lipid extracts from developing seeds of wild-type and transgenic plants were prepared as follows: between 0.5 and 1 g of seeds were heated in water at 90°C for 2 min and homogenized in 15 mL of chloroform/methanol (1:2). The lipids were extracted on a shaker for 4 h and then for 20 h with 15 mL of chloroform/methanol (2:1). The resulting organic phases were combined and then extracted with 9 mL of 0.45% NaCl, dried with Na2SO4, and evaporated under vacuum. The residue was dissolved in 1 mL of chloroform/methanol (2:1) representing the total lipid extract. Separation of lipid classes (neutral lipids, glycolipids, and phospholipids) was achieved using a Sep-pak column. Lipid extracts were loaded on the Sep-pak column pre-equilibrated with chloroform and then fractionated into the three lipid classes by elution as follows: neutral lipids with chloroform, glycolipids with acetone:isopropanol (9:1), and phospholipids with methanol. The isolation of individual components from the different lipid classes was achieved by HPTLC using a horizontal development tank (Camag, Berlin, Germany) with appropriate running solvents and standards.
Regiospecific Analysis of PC and TAG
Positional analysis of PC was conducted using the Rhizopus arrhizus lipase from Sigma as described previously (Domergue et al., 2003). The regiospecific analysis of TAGs was based on the partial degradation with Grignard reagent according to Becker et al. (1993) except that ethyl magnesium bromide (Merck, Rahway, NJ) was used instead of allyl magnesium bromide. For this purpose, 30 mg of purified TAG were dissolved in diethyl ether (1.4 mL) in a glass tube, and ethyl magnesium bromide (3 M in 100 μL of diethyl ether) was added. After 1 min of vigorous agitation, the reaction was stopped by addition of 4 mL of an acidic buffer (1 volume of 37% HCl and 36 volumes of 0.4 M boric acid solution in water). Five milliliters of diethyl ether were added, and the organic phase was washed twice with 0.4 M boric acid. After discarding the aqueous phase, the organic phase was dried with Na2SO4 and evaporated to dryness under argon without heating to avoid isomerization of the DAG. The residue was dissolved in chloroform and separated by HPTLC using chloroform-acetone (96:4 [v/v]) as solvent. The zone corresponding to 1,2-DAG and 2,3-DAG (unresolved) was scraped off the plate and extracted from the silica with chloroform/methanol (2:1 [v/v]), dried under argon without heating, and dissolved in 1 mL of anhydrous pyridine (Merck). The DAG mixture was converted to phosphatidylphenol derivatives by incubation with 100 μL of phenyldichlorophosphate (Merck) overnight at room temperature. Phosphatidylphenols were extracted according to Hajra (1974) and purified by HPTLC using chloroform/methanol/water (70:20:3 [v/v]) as solvent. After extraction from the silica (Hajra, 1974), the residue was evaporated to dryness, dissolved in 0.5 mL of diethylether, and sonicated four times for 20 s. Positional analysis was then conducted with a phospholipase A2 from bee venom (Sigma) by adding 1 mL of buffer (0.1 M boric acid, 5 mM CaCl2, and 0.1% Triton X-100, pH 8.9) and 10 μL of phospholipase A2. The mixture was incubated at 25°C for 8 h under agitation to ensure completeness of the reaction. Lipolysis products were separated by HPTLC using as solvent chloroform/methanol/aqueous ammonia (65/25/5). The spots corresponding to free fatty acids (sn-2), lyso-phosphatidylphenols (sn-1), and 2,3-phosphatidylphenols were subjected directly to transmethylation and GC analysis. Reincubation of the recovered 2,3-phosphatidylphenols with phospholipase A2 did not result in any lyso-compounds, indicating complete hydrolysis of 1,2-phosphatidylphenols. The distribution of fatty acids in the sn-3 position of TAG was deduced using the following formula: sn-3 (mol %) = [3 × TAG (mol %)] − [sn-1 (mol %)] − [sn-2 (mol %)] sn-3 (mol %) = [2 × 2,3-acyl-sn-phosphatidylphenol (mol %)] − [sn-2 (mol %)].
Analysis of PC Molecular Species
Molecular species analysis of PC was performed by electrospray tandem MS by scanning the precursors of the ions with the mass-to-charge ratio 184 in the positive mode, as previously described (Welti et al., 2002). Analyses of the acyl species in the PC species of each mass were performed by analyzing the acyl ion products of the acetate anions of the PC species (Welti et al., 2002).
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under the following accession numbers. Δ6-desaturases: PpΔ6 (AJ222980), BoΔ6 (U79010), and PtΔ6 (AY082393). Δ5-desaturases: MaΔ5 (AF054824) and PtΔ5 (AY082392). Δ6-elongases: PSE1 (AF428243) and PEA-1 (AX035537).
Supplementary Material
Acknowledgments
This research was supported financially by grants from the Bundesminesterium für Bildung and Forschung (Napus 2000, FK 0312252F) and BASF Plant Science (Ludwigshafen, Germany), which are gratefully acknowledged. We thank B. Dies, A. Fahl, and C. Ott for excellent assistance. R.W. thanks Mary Roth for technical assistance and Kansas National Science Foundation Experimental Program to Stimulate Competitive Research and Kansas Technology Enterprise Corporation for support of the Kansas Lipidomics Research Center.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Ernst Heinz (eheinz@botanik.uni-hamburg.de).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.026070.
References
- Abbadi, A., Domergue, F., Meyer, A., Riedel, K., Sperling, P., Zank, T., and Heinz, E. (2001). Transgenic oilseeds as sustainable sources of nutritionally relevant C20 and C22 polyunsaturated fatty acids? Eur. J. Lipid Sci. Technol. 103, 106–113. [Google Scholar]
- Agius, F., Gonzalez-Lamothe, R., Caballero, J.L., Munoz-Blanco, J., Botella, M.A., and Valpuesta, V. (2003). Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol 21, 177–181. [DOI] [PubMed] [Google Scholar]
- Bäumlein, H., Boerjan, W., Nagy, I., Bassuner, R., Van Montagu, M., Inze, D., and Wobus, U. (1991). A novel seed protein gene from Vicia faba is developmentally regulated in transgenic tobacco and Arabidopsis plants. Mol. Gen. Genet. 225, 459–467. [DOI] [PubMed] [Google Scholar]
- Beaudoin, F., Michaelson, L.V., Hey, S.J., Lewis, M.J., Shewry, P.R., Sayanova, O., and Napier, J.A. (2000). Heterologous reconstitution in yeast of the polyunsaturated fatty acid biosynthetic pathway. Proc. Natl. Acad. Sci. USA 97, 6421–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin, F., and Napier, J.A. (2004). Biosynthesis and compartmentation of triacylglycerol in higher plants. In Lipid Metabolism and Membrane Biogenesis, G. Daum, ed (Berlin: Springer), pp. 267–287.
- Becker, C.C., Rosenquist, A., and Holmer, G. (1993). Regiospecific analysis of triacylglycerols using allyl magnesium bromide. Lipids 28, 147–149. [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Dahlqvist, A., Stahl, U., Lenman, M., Banas, A., Lee, M., Sandager, L., Ronne, H., and Stymne, S. (2000). Phospholipid:diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerols in yeast and plants. Proc. Natl. Acad. Sci. USA 97, 6487–6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaison, L., and Moreau, D. (2002). Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: A possible mechanism of action. Cell. Mol. Life Sci. 59, 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domergue, F., Abbadi, A., Ott, C., Zank, T.K., Zähringer, U., and Heinz, E. (2003). Acyl carriers used as substrates by the desaturases and elongases involved in very long-chain polyunsaturated fatty acids biosynthesis reconstituted in yeast. J. Biol. Chem. 278, 35115–35126. [DOI] [PubMed] [Google Scholar]
- Domergue, F., Lerchl, J., Zähringer, U., and Heinz, E. (2002). Cloning and functional characterization of Phaeodactylum tricornutum front-end desaturases involved in eicosapentaenoic acid biosynthesis. Eur. J. Biochem. 269, 4105–4113. [DOI] [PubMed] [Google Scholar]
- Drexler, H., Spiekermann, P., Meyer, A., Domergue, F., Zank, T., Sperling, P., Abbadi, A., and Heinz, E. (2003. a). Metabolic engineering of fatty acids for breeding of new oilseed crops: Strategies, problems and first results. J. Plant Physiol. 160, 779–802. [DOI] [PubMed] [Google Scholar]
- Drexler, H.S., Scheffler, J.A., and Heinz, E. (2003. b). Evaluation of putative seed-specific promoters for Linum usitatissimum. Mol. Breed. 11, 149–158. [Google Scholar]
- Fiedler, U., and Conrad, U. (1995). High-level production and long-term storage of engineered antibodies in transgenic tobacco seeds. Biotechnology 13, 1090–1093. [DOI] [PubMed] [Google Scholar]
- Galili, G., Galili, S., Lewinsohn, E., and Tadmor, Y. (2002). Genetic, molecular and genomic approaches to improve the value of plant foods and feeds. Crit. Rev. Plant Sci. 21, 167–204. [Google Scholar]
- Girke, T., Schmidt, H., Zähringer, U., Reski, R., and Heinz, E. (1998). Identification of a novel Δ6-acyl-group desaturase by targeted gene disruption in Physcomitrella patens. Plant J. 15, 39–48. [DOI] [PubMed] [Google Scholar]
- Gunstone, F.D. (1992). Gamma linolenic acid occurrence and physical and chemical properties. Prog. Lipid Res. 31, 145–161. [DOI] [PubMed] [Google Scholar]
- Hajra, A.K. (1974). On extraction of acyl and alkyldihydroxyacetone phosphate from incubation mixtures. Lipids 9, 502–505. [DOI] [PubMed] [Google Scholar]
- Hites, R.A., Foran, J.A., Carpenter, D.O., Hamilton, M.C., Knuth, B.A., and Schwager, S.J. (2004). Global assessment of organic contaminants in farmed salmon. Science 303, 226–229. [DOI] [PubMed] [Google Scholar]
- Hong, H., Datla, N., Reed, D.W., Covello, P.S., MacKenzie, S.L., and Qiu, X. (2002). High-level production of gamma-linolenic acid in Brassica juncea using a Δ6 desaturase from Pythium irregulare. Plant Physiol. 129, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood, E.E., Helmer, G.L., Fraley, R.T., and Chilton, M.D. (1986). The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J. Bacteriol. 168, 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazu, C.E., Gonzalez-Rodriguez, S., and Brenner, R.R. (1993). Delta 5 desaturase activity in rat kidney microsomes. Mol. Cell. Biochem. 129, 31–37. [DOI] [PubMed] [Google Scholar]
- Jongsma, M.A., Bakker, P.L., Peters, J., Bosch, D., and Stiekema, W.J. (1995). Adaptation of Spodoptera exigua larvae to plant proteinase inhibitors by induction of gut proteinase activity insensitive to inhibition. Proc. Natl. Acad. Sci. USA 92, 8041–8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump, D.B. (2002). The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 277, 8755–8758. [DOI] [PubMed] [Google Scholar]
- Larson, T.R., Edgell, T., Byrne, J., Dehesh, K., and Graham, I.A. (2002). Acyl CoA profiles of transgenic plants that accumulate medium-chain fatty acids indicate inefficient storage lipid synthesis in developing oilseeds. Plant J. 32, 519–527. [DOI] [PubMed] [Google Scholar]
- Larson, T.R., and Graham, I.A. (2001). Technical advance: A novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J. 25, 115–125. [DOI] [PubMed] [Google Scholar]
- Liu, J.W., Huang, Y.S., DeMichele, S., Bergana, M., Bobik, E., Hastilow, C., Chuang, L.T., Mukerji, P., and Knutzon, D. (2001). Evalution of the seed oil from a canola plant genetically transformed to produce high levels of gamma-linolenic acid. In Gamma-Linolenic Acid: Recent Advances in Biotechnology and Clinical Applications, Y.S. Huang and A. Ziboh, eds (Champaign, IL: AOCS Press), pp. 61–71.
- Lopez Alonso, D., and Garcia Maroto, F. (2000). Plants as chemical factories for the production of polyunsaturated fatty acids. Biotechnol. Adv. 18, 481–497. [DOI] [PubMed] [Google Scholar]
- Mattson, F.H., and Volpenhein, R.A. (1963). The specific distribution of unsaturated fatty acids in the triaglycerides of plants. J. Lipid Res. 4, 392–396. [PubMed] [Google Scholar]
- Michaelson, L.V., Lazarus, C.M., Griffiths, G., Napier, J.A., and Stobart, A.K. (1998). Isolation of a Delta5-fatty acid desaturase gene from Mortierella alpina. J. Biol. Chem. 273, 19055–19059. [DOI] [PubMed] [Google Scholar]
- Napier, J.A., Hey, S.J., Lacey, D.J., and Shewry, P.R. (1998). Identification of a Caenorhabditis elegans Δ6-fatty-acid-desaturase by heterologous expression in Saccharomyces cerevisiae. Biochem. J. 330, 611–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu, T., Nagao, M., Ishibashi, T., and Imai, Y. (1981). Purification and partial characterization of linoleoyl-CoA desaturase from rat liver microsomes. Arch. Biochem. Biophys. 206, 21–28. [DOI] [PubMed] [Google Scholar]
- Okuyama, H., Kobayashi, T., and Watanabe, S. (1996). Dietary fatty acids–the N-6/N-3 balance and chronic elderly diseases. Excess linoleic acid and relative N-3 deficiency syndrome seen in Japan. Prog. Lipid Res. 35, 409–457. [DOI] [PubMed] [Google Scholar]
- Pauly, D., Christensen, V., Guenette, S., Pitcher, T.J., Sumaila, U.R., Walters, C.J., Watson, R., and Zeller, D. (2002). Towards sustainability in world fisheries. Nature 418, 689–695. [DOI] [PubMed] [Google Scholar]
- Qi, B., Beaudoin, F., Fraser, T., Stobart, A.K., Napier, J.A., and Lazarus, C.M. (2002). Identification of a cDNA encoding a novel C18-Δ9 polyunsaturated fatty acid-specific elongating activity from the docosahexaenoic acid (DHA)-producing microalga, Isochrysis galbana. FEBS Lett. 510, 159–165. [DOI] [PubMed] [Google Scholar]
- Qi, B., Fraser, T., Mugford, S., Dobson, G., Sayanova, O., Butler, J., Napier, J.A., Stobart, A.K., and Lazarus, C.M. (2004). Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat. Biotechnol 22, 739–749. [DOI] [PubMed] [Google Scholar]
- Roughan, P.G., and Slack, C.R. (1982). Cellular organization of glycerolipid metabolism. Annu. Rev. Plant Physiol. 33, 97–132. [Google Scholar]
- Sayanova, O., Smith, M.A., Lapinskas, P., Stobart, A.K., Dobson, G., Christie, W.W., Shewry, P.R., and Napier, J.A. (1997). Expression of a borage desaturase cDNA containing an N-terminal cytochrome b5 domain results in the accumulation of high levels of Δ6-desaturated fatty acids in transgenic tobacco. Proc. Natl. Acad. Sci. USA 94, 4211–4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani, D., and DellaPenna, D. (1998). Elevating the vitamin E content of plants through metabolic engineering. Science 282, 2098–2100. [DOI] [PubMed] [Google Scholar]
- Slack, C.R., Campbell, C.C., Browse, J., and Roughan, P.G. (1983). Some evidence for the reversibility of the cholinephosphotransferase-catalyzed reaction in developing linseed cotyledons in vivo. Biochim. Biophys. Acta 754, 10–20. [Google Scholar]
- Slack, C.R., Roughan, P.G., and Balasingham, N. (1978). Labelling of glycerolipids in the cotyledons of developing oilseeds by [1-14C] acetate and [2-3H] glycerol. Biochem. J. 170, 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville, C., and Browse, J. (1991). Plant Lipids: Metabolism, mutants and membranes. Science 252, 80–87. [DOI] [PubMed] [Google Scholar]
- Sperling, P., Lee, M., Girke, T., Zähringer, U., Stymne, S., and Heinz, E. (2000). A bifunctional Δ6-fatty acyl acetylenase/desaturase from the moss Ceratodon purpureus. A new member of the cytochrome b5 superfamily. Eur. J. Biochem. 267, 3801–3811. [DOI] [PubMed] [Google Scholar]
- Sprecher, H. (2002). The roles of anabolic and catabolic reactions in the synthesis and recycling of polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 67, 79–83. [DOI] [PubMed] [Google Scholar]
- Stobart, A.K., Mancha, M., Lenman, M., Dahlqvist, A., and Stymne, S. (1997). Triacylglycerols are synthesised and utilized by transacylation reactions in microsomal preparations of developing safflower (Carthamus tinctorius L.) seeds. Planta 203, 58–66. [Google Scholar]
- Stymne, S., and Stobart, A.K. (1984). Evidence for the reversibility of the acyl-CoA:lysophosphatidylcholine acyltransferase in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons and rat liver. Biochem. J. 223, 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne, S., and Stobart, A.K. (1986). Biosynthesis of gamma-linolenic acid in cotyledons and microsomal preparations of the developing seeds of common borage (Borago officinalis). Biochem. J. 240, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne, S., Stobart, A.K., and Glad, G. (1983). The role of the acyl-CoA pool in the synthesis of polyunsaturated 18-carbon fatty acids and triacylglycerol production in the microsomes of developing safflower seeds. Biochim. Biophys. Acta 752, 198–208. [DOI] [PubMed] [Google Scholar]
- Thomas, T.L. (1993). Gene expression during plant embryogenesis and germination: An overview. Plant Cell 5, 1401–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein, E.A. (2001). n-3 Fatty acids—Physiological and technical aspects for their use. Eur. J. Lipid Sci. Technol. 103, 45–55. [Google Scholar]
- Voelker, T., and Kinney, A.J. (2001). Variations in the biosynthesis of seed-storage lipids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 335–361. [DOI] [PubMed] [Google Scholar]
- Vogel, G., and Browse, J. (1996). Cholinephosphotransferase and diacylglycerol acyltransferase. Plant Physiol. 110, 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis, J.G., and Browse, J. (1999). The Δ8-desaturase of Euglena gracilis: An alternate pathway for synthesis of 20-carbon polyunsaturated fatty acids. Arch. Biochem. Biophys. 365, 307–316. [DOI] [PubMed] [Google Scholar]
- Welti, R., Li, W., Li, M., Sang, Y., Biesiada, H., Zhou, H.-E., Rajashekar, C.B., Williams, T.D., and Wang, X. (2002). Profiling membrane lipids in plant stress response. J. Biol. Chem. 277, 31994–32002. [DOI] [PubMed] [Google Scholar]
- Ye, X., Al-Babili, S., Kloti, A., Zhang, J., Lucca, P., Beyer, P., and Potrykus, I. (2000). Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287, 303–305. [DOI] [PubMed] [Google Scholar]
- Zank, T.K., Zähringer, U., Beckmann, C., Pohnert, G., Boland, W., Holtorf, H., Reski, R., Lerchl, J., and Heinz, E. (2002). Cloning and functional characterisation of an enzyme involved in the elongation of Δ6-polyunsaturated fatty acids from the moss Physcomitrella patens. Plant J. 31, 255–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.