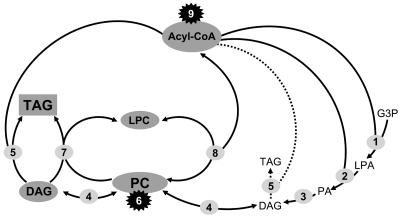
Figure 10.
Accumulation of Linseed TAG by Channeling of Acyl Groups through PC.
The stepwise acylation of glycerol-3-phosphate (G3P) by acyl-CoA:1-glycerol-3-phosphate acyltransferase (G3PAT; 1) and acyl-CoA:1-acyl-glycerol-3-phosphate acyltransferase (2) yields 1-acyl-glycerol-3-phosphate (LPA) and phosphatidic acid (PA), which is hydrolyzed by phosphatidic acid phosphatase (3) to give DAG. Its conversion to TAG by acyl-CoA:diacylglycerol acyltransferase (5) immediately after the primary formation of DAG by the Kennedy pathway represents a minor alternative for TAG synthesis in linseed (indicated by the dotted arrow). The major part of DAG is converted to PC in a reversible reaction catalyzed by CDP-choline:diacylglycerol cholinephosphotransferase (CPT; 4). PC is the substrate for acyl group desaturases (6). They may accept both the sn-1 and sn-2–localized acyl groups (the genuine enzymes), or their action may be confined to the sn-2–bound acyl chains (the heterologously expressed Δ6- and Δ5-desaturase). In a reversible reaction, the acyl-CoA:lyso-phosphatidylcholine acyltransferase (8) equilibrates the sn-2–bound acyl group with the acyl-CoA pool. The formation of C20-polyunsaturated fatty acids by the heterologously expressed acyl-CoA elongase (9) occurs by elongation of Δ6-C18–polyunsaturated acyl-CoA and thus depends on their release from PC into the acyl-CoA pool by the LPCAT (8). The activities of the desaturases (6) and elongase (9) together with the reversibility of LPCAT (8) and CPT (4) result in a continuous change of the acyl groups of the acyl-CoA pool, PC, and DAG, which all participate in this equilibration. Only after passage through PC, a highly desaturated DAG is finally converted into TAG. This channeling is catalyzed by two enzymes, the DAGAT (5) mentioned before and the phospholipid:diacylglycerol acyltransferase (PDAT; 7) that transfers the sn-2–bound acyl group from PC to the sn-3 position of TAG. These major routes, deduced by labeling studies with wild-type linseed (Slack et al., 1983; Stymne and Stobart, 1984), are supported by the acyl profiles of the various pools (acyl-CoA, PC, DAG, and TAG) studied in this investigation with transgenic linseed.