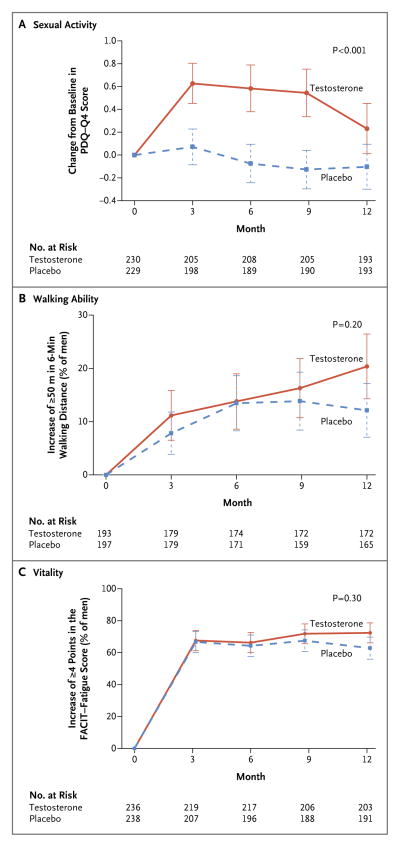
Figure 1. Primary Outcomes in the Three Main Trials of the Testosterone Trials.
The primary outcome of the Sexual Function Trial (Panel A) was the change from baseline in the score for sexual activity (question 4) on the Psychosexual Daily Questionnaire (PDQ-Q4; range, 0 to 12, with higher scores indicating more activity). The primary outcome of the Physical Function Trial (Panel B) was the percentage of men who had an increase of at least 50 m in the distance walked during the 6-minute walk test. The primary outcome of the Vitality Trial (Panel C) was the percentage of men who had an increase of at least 4 points in the score on the Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue scale (range, 0 to 52, with higher scores indicating less fatigue). P values were calculated with the use of a linear random-effects model for sexual activity and logistic random-effects models for walking ability and vitality. The I bars represent standard deviations.