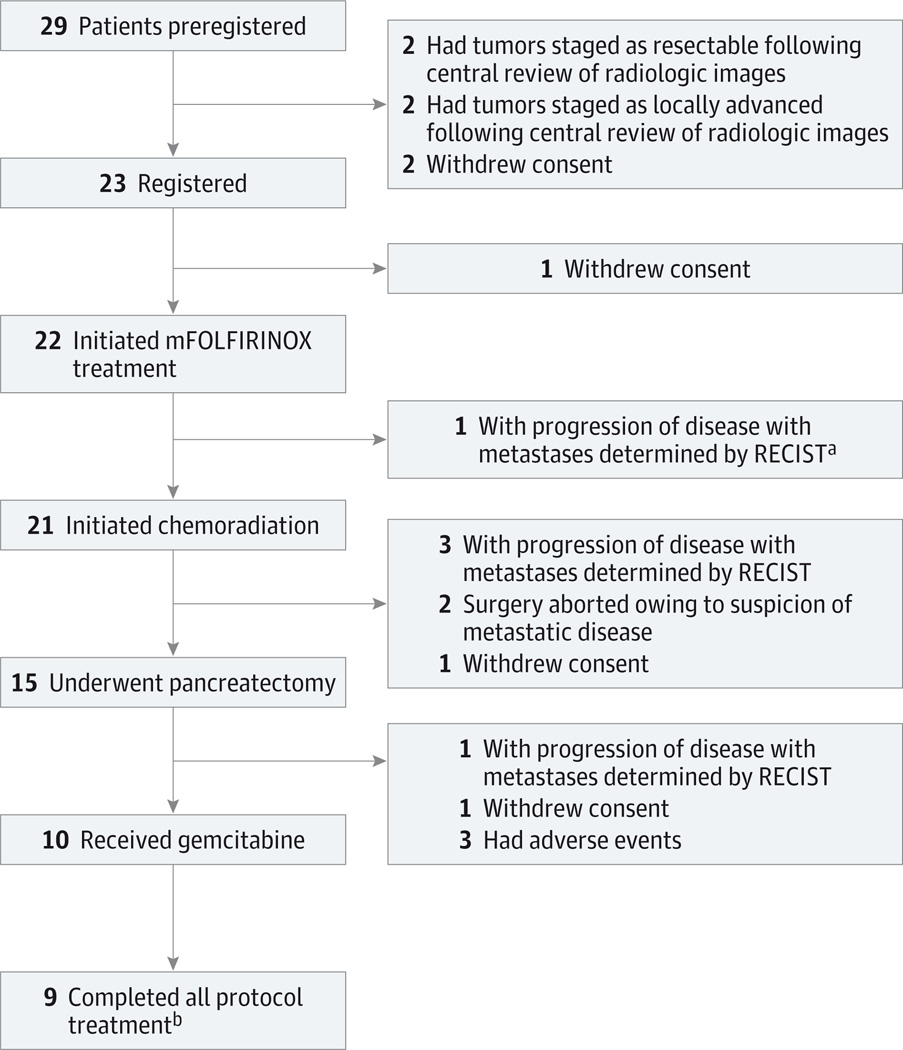
mFOLFIRINOX indicates modified treatment with 85 mg/m
2 of oxaliplatin, 180 mg/m
2 of irinotecan hydrochloride, 400 mg/m
2 of leucovorin calcium, and then 2400 mg/m
2 of 5-fluorouracil for 4 cycles; RECIST, Response Evaluation Criteria in Solid Tumors.
An additional patient had progression of disease determined by RECIST owing to isolated progression after mFOLFIRINOX treatment but, per protocol, proceeded to chemoradiation.
Treatment was halted for 1 patient after first cycle of postoperative chemotherapy.