Abstract
The US Food and Drug Administration has now approved 10 direct-acting antivirals (DAAs) for the management of hepatitis C virus (HCV). These therapies are combined into 6 regimens that are given for varying durations, with or without ribavirin, depending on the viral genotype, the presence or absence of baseline resistance-associated variants (RAVs), and the patient type. RAVs may be present before exposure to a drug or may become detectable de novo during exposure to a drug. Emerging resistant strains are the most common cause of failure of HCV DAA regimens. Second-generation DAAs provide superior coverage of resistant variants compared with first-generation members of that class. They may also cover a broader range of viral genotypes. Numerous clinical trials have evaluated the safety and efficacy of DAAs in a variety of patient populations, including those with cirrhosis, HIV, and end-stage renal disease. This article evaluates the data from these studies, and discusses recommendations from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) guidance document.
Introduction
The current approach to treatment of hepatitis C virus (HCV) is based on targeting several HCV proteins. As of September 2016, the approach includes 10 approved direct-acting antivirals (DAAs) in 3 classes. These DAAs are combined into 6 regimens that are given for varying durations, and sometimes with ribavirin, depending upon the viral genotype (GT), the presence or absence of baseline resistance-associated variants (RAVs), and the patient type. Specifically, the US Food and Drug Administration (FDA) has approved 3 NS3-4A protease inhibitors (simeprevir [SMV], paritaprevir [PTV], and grazoprevir [GZR]), 5 NS5A inhibitors (ledipasvir [LDV], ombitasvir [OBV], daclatasvir [DCV], elbasvir [EBR], and velpatasvir [VEL]), and 2 NS5B polymerase inhibitors (the nucleotide analog sofosbuvir [SOF] and the non-nucleoside analog dasabuvir [DSV]) for the management of HCV.
Throughout this supplement, references are made to the HCV guidance document from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA).1 The information is based on the September 16, 2016 version, which is available at www.hcvguidelines.org.
The Impact of HCV RAVs in GT1 Patients
When the genetic variants of a virus have less sensitivity to a DAA drug than the wild-type virus for which the drug was designed, the variants are referred to as resistance-associated variants, or RAVs (alternative designations in use include resistance-associated polymorphisms [RAPs] and resistance-associated substitutions [RASs]). Variants may be present at detectable levels within a patient’s viral population before exposure to a drug or may become detectable de novo during exposure to a drug. The emergence of viral resistance to a drug during a course of antiviral therapy is not caused by the drug(s); the resistant variant(s) are generated during the natural process of viral replication and may be selected because of suppression from competing viral strains that are susceptible to the drug(s). Emerging resistant strains are the most common cause of failure of HCV DAA regimens. Advanced, or second-generation, DAAs provide superior coverage of resistant variants compared with first-generation members of that class. They may also cover a broader range of viral GTs.
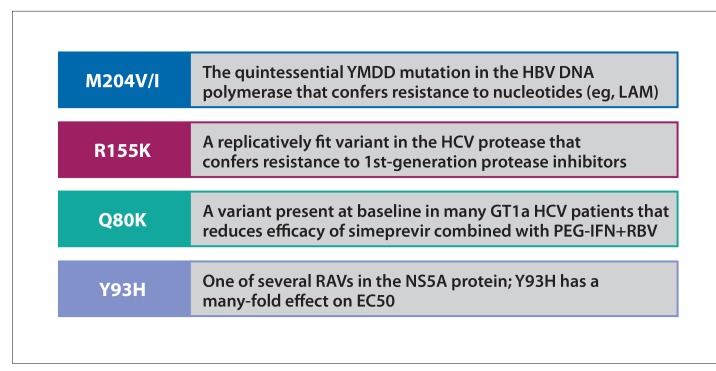
The 4 prominent examples of RAVs in viral hepatitis that show the nomenclature of resistance are depicted in Figure 1. HCV resistance was not an issue with interferon (IFN)-based regimens before the introduction of DAAs. When using peginterferon (PEG-IFN) and protease inhibitors, patients with virologic failure often developed RAVs. Virologic failure is more common in GT1a than 1b because of differences in the barrier to resistance for key RAVs or, in certain cases, potency for the protease and NS5A inhibitor classes. For example, GT1b RAVs associated with protease inhibitors need 2 nucleotide substitutions in 2 critical codons, whereas GT1a RAVs need only one. The Q80K polymorphism in the protease domain is associated with reduced sensitivity to the protease inhibitor simeprevir, a discovery that led to the first recommendation for baseline testing of RAVs in patients with GT1a who are receiving this agent with peginterferon and ribavirin.
Figure 1.
“Famous” RAVs in viral hepatitis.
GT, genotype; H, histamine; HBV, hepatitis B virus; HCV, hepatitis C virus; I, isoleucine; K, lysine; M, methionine; PEG-IFN, peginterferon; Q, glutamine; R, arginine; RAVs, resistance-associated variants; RBV, ribavirin; V, valine; Y, tyrosine.
Figure courtesy of Dr Ira M. Jacobson.
RAVs associated with protease inhibitors, NS5A, or non-nucleotide NS5B often accompany virologic failure with interferon-free DAA regimens, depending on the drugs included in the regimen. RAVs associated with NS5A persist longer than those associated with protease inhibitors, and can lead to the failure of treatment with a regimen containing an NS5A inhibitor. This poses a challenge for retreatment with NS5A inhibitors that share the resistance profile of the prior agent. Nucleotide polymerase inhibitors have the highest barrier to resistance because of the poor replicative fitness of the “signature” in vitro RAV (S282T).
The prevalence of pretreatment NS5A RAVs in GT1 patients and their effect on treatment outcomes associated with the use of LDV/SOF were assessed in 3 trials: ION-1 (treatment-naive patients with and without cirrhosis),2 ION-2 (interferon-experienced patients with and without cirrhosis),3 and ION-3 (treatment-naive patients without cirrhosis).4 Between 14% to 18% of patients in these trials had baseline NS5A RAVs detected. In ION-1, treatment-naive, noncirrhotic patients with baseline NS5A RAVs had slightly lower rates of sustained virologic response at week 12 (SVR12) than those without baseline NS5A RAVs (96% vs 99%, respectively).2 From an analysis of over 5000 patients gleaned from various LDV/SOF studies, treatment-experienced patients with cirrhosis had lower SVR12 rates if baseline NS5A RAVs were detected. Treatment with 12 weeks of LDV/SOF plus RBV led to SVR12 rates of 89% among patients with NS5A RAVs vs 96% among those without. After treatment with 24 weeks of LDV/SOF, SVR12 rates were 87% in patients with baseline RAVs vs 100% in those without.5 Among treatment-experienced noncirrhotic patients, the presence of baseline RAVs decreased SVR12 (achieved by 90% in patients with RAVs vs 99% in patients without RAVs).
A recent analysis of baseline samples from 2144 phase 2 and 3 LDV/SOF study participants demonstrated that baseline RASs in the NS5A region had no impact on SVR12 in GT1b patients, but had a small effect on GT1a patients in the treatment-experienced cohort. In addition, 8 weeks of LDV/SOF in treatment-naive, noncirrhotic patients with a baseline viral load of less than 6 million IU/mL was not affected by RAVs with less than 100-fold resistance to ledipasvir, but was impacted by RAVs with more than 100-fold resistance to ledipasvir.6 However, this finding appeared to be confined to treatment-naive, noncirrhotic patients with HCV RNA higher than 6 million IU/mL, in whom 8 weeks of treatment is not indicated in any event. There are no guidelines recommending baseline RAV testing with LDV/SOF.
The ASTRAL-1 study in 624 patients, including 328 with GT1, assessed a 12-week regimen of VEL 100 mg/SOF 400 mg in treatment-naive and treatment-experienced patients with GT1, 2, 4, 5, or 6.7 Overall SVR was 99%, and baseline NS5A RAVs did not influence the SVR12 rate for GT1 patients. The only virologic failures were in 2 GT1 patients.7 In another study of SOF/VEL plus RBV for 24 weeks among patients with prior NS5A inhibitor exposure, many of whom had baseline RAVs, nearly all patients with GT1 (33 of 34 [97%]) had an SVR12.8 This 24-week regimen is not presently approved.
The C-EDGE study of treatment-naive patients assessed a 12-week regimen of oral, once-daily, fixed-dose GZR 100 mg/EBR 50 mg in GT1, 4, and 6.9 SVR12 was high for all patients (95%), whether with cirrhosis (97%) or without (94%). GT1a-infected patients had lower SVR12 (92%) compared with those with GT1b (99%). Among the 9 GT1a patients with baseline NS5A RAVs and resistance against EBR, only 2 patients (22%) achieved SVR12. In the C-EDGE study of treatment-experienced patients, GZR/EBR with or without RBV for 12 or 16 weeks was assessed for efficacy and safety in patients who had previously failed PEG-IFN plus RBV therapy.10 Patients not receiving RBV had lower rates of SVR12 (92% for both 12 and 16 weeks) when compared with patients who received RBV (94% and 97% for 12 and 16 weeks, respectively). When SVR12 was assessed based on baseline NS3 and NS5 RAVs, researchers determined that SVR12 was highly impacted in GT1a patients with baseline NS5A RAVs with a greater than 5-fold shift (52%; reflecting substitutions at the M28, Q30, L31, and/or Y93 positions).
In January 2016, GZR/EBR became the first NS5A-containing regimen for which baseline RAV testing was indicated, a stipulation specific for GT1a patients. In patients with RAVs detected in any of the 4 positions (M28, Q30, L31, or Y93), therapy extension and the addition of ribavirin is recommended because these RAVs reduce the rate of SVR if the regimen is given for the otherwise standard 12 weeks without ribavirin. This modified regimen is independent of the presence of cirrhosis and whether or not the patient had already received treatment. However, it is not necessary in patients with GT1b, in whom SVR appears to be unaffected by baseline RAVs.
Although RBV appears to work with DAAs by helping to control resistant variants, the mechanisms underlying this effect remain unclear. A theory for RBV’s mechanism of action in HCV involves mutagenesis and “error catastrophe” by generation of defective genomes with impaired fitness. RAVs in the setting of NS5A or protease inhibitors are generally less fit. The generation of NS5A/protease inhibitor RAVs in the setting of RBV-associated defective genomes may produce an additive or synergistic effect and lead to less viral fitness than either alone.
To summarize the recommendations pertaining to baseline RAVs in the AASLD/IDSA guidelines1: For patients who are DAA–treatmentnaive, the AASLD/IDSA guidelines do not recommend resistance testing when using LDV/SOF, PTV/ritonavir(r)/OBV plus DSV, or DCV plus SOF. If the GZR/EBR regimen is selected, baseline testing is suggested for HCV GT1a, independent of previous treatment with PEG-IFN plus RBV and cirrhotic status. GZR/EBR treatment for 12 weeks is recommended if no high-level RAVs (M28, Q30, L31, or Y93) are detected, and GZR/EBR plus RBV for 16 weeks is designated an “alternative” treatment by the guidelines if these RAVs are present. A baseline test for Q80K in HCV GT1a-infected cirrhotic patients is recommended when considering treatment with SMV/SOF. Following failure with SMV/SOF, treatment should be deferred in noncirrhotic patients or others who lack urgent need, and patients should be tested for RAVs to NS3 protease inhibitors and NS5A inhibitors. If a nucleotide-based dual therapy is chosen, treat for 24 weeks with RBV; however, consider nucleotide-based triple or quadruple regimens for 12 to 24 weeks with RBV if available. These recommendations all apply to patients previously treated with NS5A inhibitors.
Data are beginning to appear on the use of investigational regimens with advanced NS5A and protease inhibitors for DAA failures. For example, the MAGELLAN study evaluated the investigational combination of ABT-493, a second-generation protease inhibitor, and ABT-530, a second-generation NS5A inhibitor, in 50 such GT1 patients.11 Baseline RAVs were present in 41 of the 50 patients (81%). Fifteen of the patients had only NS3 RAVs (30%), 10 had only NS5A RAVs (20%), and 16 (32%) had both. The 3 dosing arms consisted of: 200-mg ABT-493 plus 80-mg ABT-530; 300-mg ABT-493 plus 120-mg ABT-530 plus RBV; and, lastly, 300-mg ABT-493 plus 120-mg ABT-530. The SVR12 for these 3 groups was 100%, 95%, and 95%, respectively. Only 2 patients failed therapy, and both were virologic failures (1 relapse and 1 breakthrough).
Another new triplet regimen in development consists of SOF/VEL/GS-9857 (a second-generation protease inhibitor), with or without ribavirin. This combination was studied for 12 weeks in 63 DAA-experienced GT1 patients. Many of these patients had single- or multiple-class RAVs, and patients with cirrhosis were included. Of the 63 patients, 100% achieved SVR12.12
Both of the above-described triplet regimens have also shown promising results in non–DAA-exposed populations, as has another triplet regimen, GZR/MK3682/MK8408.13 No results are yet available for the latter regimen for DAA-failure patients.
Treatment of Non-GT1 HCV-Infected Patients
SOF 400 mg/VEL 100 mg dosed for 12 weeks was recently approved as the first DAA combination for all HCV genotypes (GT1-6). The fixed-dose combination tablet is dosed once daily and can be used in patients with compensated cirrhosis (Child-Pugh A). In patients with decompensated cirrhosis (Child-Pugh B and C), ribavirin should be added to the regimen.
Genotype 2
ASTRAL-2 studied 12-week regimens of SOF/VEL vs the prevailing standard of care, SOF plus RBV, in GT2-infected patients.14 Rates of SVR12 were 99% among GT2 patients treated with SOF/VEL compared with 94% among those treated with SOF plus RBV. This study established SOF/VEL as the recommended regimen for GT2 treatment-naive and treatment-experienced patients with or without compensated cirrhosis, supplanting SOF plus RBV.
Although daclatasvir (60 mg) plus SOF (400 mg) is not FDA-approved for GT2-infected patients, the AASLD/IDSA guidance document states that it may be considered an alternative treatment for GT2-infected patients without cirrhosis or with compensated cirrhosis based on limited data from an earlier trial.1,15 Patients without cirrhosis can be treated for 12 weeks, while those with compensated cirrhosis need their treatment duration extended to 16 to 24 weeks.
Genotype 3
The treatment of GT3 has been associated with significantly higher risks of cirrhosis and hepatocellular carcinoma as compared with HCV GT1. This observation is based on data from the Veterans Health Administration HCV Clinical Case Registry between the years 2000 and 2009. The majority (80%) of the 109,762 cases were GT1, followed by GT2 (12%), and GT3 (7.5%). The mean duration of patient follow-up was approximately 5 and a half years. After adjustment for demographic, clinical, and antiviral treatment factors, GT3 showed an increased risk for both cirrhosis (hazard ratio [HR], 1.31; 95% confidence interval [CI], 1.22-1.39) and hepatocellular carcinoma (HR, 1.80; 95% CI, 1.61-2.03) compared with GT1.16 One proposed explanation for this association of HCV GT3 with pathogenicity is the frequent accumulation of fat in the hepatocytes of GT3–infected patients—a viral effect rather than a manifestation of the mechanistic pathways comprising metabolically based fatty liver disease.
In the ASTRAL-3 trial, the SVR12 for SOF/VEL dosed for 12 weeks was superior to SOF plus RBV dosed for 24 weeks in GT3 patients (95% vs 80%, respectively).14 Among treatment-naive, noncirrhotic patients, SVR12 was 98% with SOF/VEL given for 12 weeks vs 90% for those treated with SOF plus RBV for 24 weeks. Treatment-naive, cirrhotic patients also had higher SVR12 rates with the SOF/VEL regimen than with SOF plus RBV (93% vs 73%, respectively). SVR12 was achieved by 91% of treatment-experienced cirrhotic patients, and that increased to 94% when the patient who was reinfected was excluded. In the resistance analysis, 16% of the patients had baseline NS5A RAVs. SVR12 was 88% in those with baseline NS5A RAVs vs 97% in those without baseline RAVs. SVR12 rates with SOF/VEL were slightly lower in GT3 treatment-experienced patients, at 90%. GT3 patients who were treatment-experienced and cirrhotic had an 89% SVR12 rate.
The ALLY-3 study examined treatment-naive and treatment-experienced GT3 patients treated with DCV/SOF for 12 weeks. Eighty-nine percent of patients achieved SVR12,17 including 96% of patients without cirrhosis. However, SVR12 rates for treatment-naive and treatment-experienced patients with compensated cirrhosis were 58% and 69%, respectively. The subsequent ALLY-3+ study examined GT3 patients treated with DCV/SOF plus RBV for 12 and 16 weeks.18 Ninety percent of patients achieved SVR12. The SVRs for GT3 patients and treatment-experienced patients with compensated cirrhosis were 88% and 86% for DCV/SOF plus RBV in the 12-week and 16-week arms, respectively. Even though the FDA-approved label recommends 12 weeks of therapy, additional clinical data from Europe support treatment with DCV plus SOF with RBV for up to 24 weeks in treatment-experienced cirrhotic patients. This longer duration is recommended in the AASLD/IDSA and EASL guidance documents.
The AASLD/IDSA guidance document recommends the following management strategies1:
Treatment-naive without cirrhosis: SOF/VEL × 12 weeks or DCV plus SOF × 12 weeks.
-
Treatment-naive with cirrhosis: SOF/VEL × 12 weeks or DCV plus SOF with or without RBV × 24 weeks.
– RAV testing is recommended; add RBV if Y93H is present.
-
Treatment-experienced without cirrhosis: SOF/VEL × 12 weeks or DCV plus SOF × 12 weeks.
– RAV testing is recommended; add RBV if Y93H is present.
Treatment-experienced with cirrhosis: SOF/VEL plus RBV × 12 weeks or DCV plus SOF plus RBV × 24 weeks.
SOF plus RBV failures: SOF/VEL plus RBV × 12 weeks or DCV plus SOF plus RBV × 24 weeks.
Genotype 4
HCV GT4 accounts for approximately 1% to 2% of HCV infections in the United States. It is more commonly observed in Egypt, Saudi Arabia, North Africa, and Southern Europe. Historically, SVR rates with IFN-based therapy have been comparable between patients with GT1 and GT4. Four studies have focused on the rates of SVR12 associated with different regimens in treatment-naive GT4 patients.7,19-21 The results all demonstrate high SVRs for patients treated for 12 weeks with either SOF/VEL (100%), EBV/GZR (100%), SOF/LDV (99%), and PTV/r/OBV plus RBV (100%).
Genotypes 5 and 6
HCV GTs 5 and 6 are less commonly observed GTs in the United States. There are limited data on treatment of these GTs with DAA therapy, and only SOF/VEL and SOF/LDV for 12 weeks have been approved by the FDA in these settings. SOF/LDV dosed for 12 weeks resulted in an SVR of 95% in patients with GT5,22 and SOF/VEL dosed for 12 weeks resulted in an SVR12 of 100% in ASTRAL-1 among patients with GT5 and GT6.7 The all-oral, 12-week, pangenotypic regimen of SOF/VEL—which is associated with SVR12 rates exceeding 95%—is now approved for genotypes 1 through 6.23
All-oral doublet or triplet pangenotypic regimens, such as those described earlier, are in late-stage development and may offer advantages, such as shorter duration and successful retreatment of patients who failed therapy with first-generation DAAs. High levels of efficacy across several genotypes, including GT3, have been described in phase 2 studies.
HIV/HCV Coinfection
Chronic HCV infections are common among persons living with HIV, with an overall prevalence of approximately 25%. The prevalence differs by HIV risk group, with coinfection seen in 65% of patients who inject drugs, 15% of patients who engage in heterosexual sex, and 8% of men who have sex with men.24,25 In a systematic review and meta-analysis of HCV sexual transmission among men who have sex with men, HCV seroconversion increased from an estimated rate of 0.42 per 100 person-years in 1999, to 1.09 per 100 person-years in 2010, to 1.34 per 100 person-years in 2012.26 Infections were attributable to high-risk behaviors, including traumatic sex and sex while on methamphetamines. A report on an HIV outbreak in Scott County, Indiana from 2015 to 2016 found a relationship between the outbreak and the use of injection narcotics, with more than 90% of the newly diagnosed HIV patients showing HCV coinfection by early 2016.27
Liver disease remains a major cause of death in HIV-infected persons, accounting for 13% of all HIV-related deaths.28 Despite the availability of effective antiretroviral therapies (ARTs), HCV disease progression remains faster in HIV/HCV-coinfected patients compared with HCV-monoinfected patients.29 For example, ART-treated coinfected patients with HIV RNA below 1000 copies/mL have a 65% excess risk of hepatic decompensation within 10 years, while those with HIV RNA at or above 1000 copies/mL have an 82% excess risk. Similarly, those with CD4 below 200/mm2 have a 203% excess risk for the same outcome, while those with CD4 at or above 200/mm2 have a 56% to 63% excess risk. Further, the results of the NA-ACCORD study showed that incident end-stage liver disease (ESLD) in HIV-infected adults assessed between 2000 and 2009 was attributed to HCV, as was alcohol use and CD4 below 200/mm2.30 In that study, the population attributable fraction—which is the proportion of ESLD that could be avoided if the at-risk persons were not exposed to the modifiable factor—for HCV was 33%. SVR has been associated with a substantial reduction in mortality for the general population, patients with cirrhosis, and patients with HIV coinfection.31 After adjusting for confounding factors, SVR was associated with a decreased risk of all-cause mortality for the general HCV-infected population (50% reduction), cirrhotic patients (74% reduction), and coinfected patients (79%).31
Several trials have examined various treatment regimens for HIV/HCV coinfection. The ALLY-2 trial was an open-label efficacy and safety study of the combination of DCV/SOF in 151 treatment-naive and 52 treatment-experienced patients with HIV/HCV-coinfection.32 Patients with HCV who had not received previous treatment were randomized 2:1 to receive DCV at a standard dose of 60 mg daily (with dose adjustment for concomitant ART to 30 mg in patients receiving ritonavir-boosted protease inhibitors and to 90 mg in those receiving efavirenz or nevirapine) in addition to daily doses of 400 mg SOF for 12 weeks or 8 weeks. The HCV treatment-experienced patients received 12 weeks of therapy at the same doses. The majority of patients were GT1a (70% in the treatment-naive cohort and 63% in the treatment-experienced cohort) or GT1b (12% in the treatment-naive cohort and 21% in the treatment-experienced cohort). Patients with cirrhosis were also included. SVR12 for GT1 patients in the 12-week treatment-naive and treatment-experienced arms were 96% and 98%, respectively, as opposed to 76% in the 8-week arm.
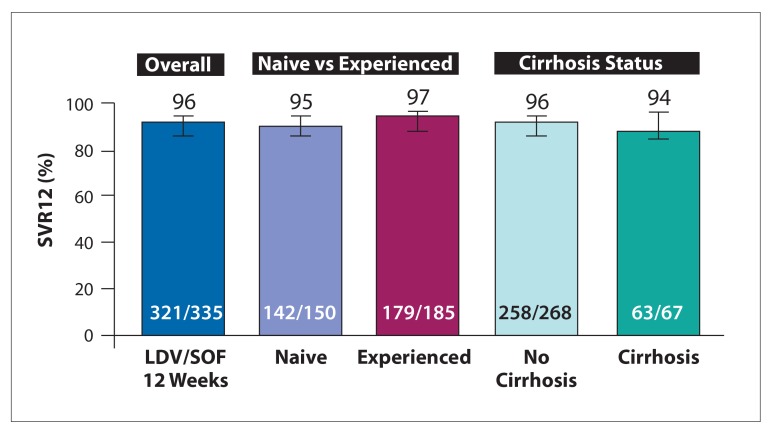
The ION-4 study was a multicenter, single-arm, open-label study testing 12 weeks of LDV/SOF in 335 HIV patients coinfected with HCV GTs 1 or 4.33 The 335 enrolled patients all received ART. The study included treatment-naive and treatment-experienced patients, as well as patients with cirrhosis. The SVR12 rates for all patients are shown in Figure 2. High rates of SVR12 were achieved in the population overall (96%), including those with cirrhosis (94%). African American patients had lower SVR12 rates compared with non–African Americans (90% vs 99%, respectively). Multivariate logistic regression demonstrated an association between relapse and black race, IL28B TT, and use of efavirenz.
Figure 2.
SVR12 in HCV/HIV-coinfected patients with genotype 1 infection treated with ledipasvir/sofosbuvir for 12 weeks.
HCV, hepatitis C virus; HIV, human immunodeficiency virus; LDV/SOF, ledipasvir/sofosbuvir; SVR12, sustained virologic response at week 12. Adapted from Naggie S et al. N Engl J Med. 2015;373(8):705-713.33
The TURQUOISE-I trial assessed the efficacy and safety of 12 or 24 weeks of PTV/r/OMV plus DSV plus RBV in HIV/HCV GT1 coinfected patients with or without cirrhosis.34 The 63 treatment-experienced or treatment-naive patients were on stable ART regimens. SVR12 rates after treatment for 12 and 24 weeks were 94% and 91%, respectively. Virologic failure was reported in 2 patients with HCV GT1a, cirrhosis, and prior null response to PEG-IFN plus RBV (1 in each study arm). These studies observed 2 cases of HCV reinfection in the 24-week treatment arm.
More recently, the results from part 1b of TURQUOISE-I were reported. This part of the study examined HIV/HCV GT1 coinfected patients on stable darunavir-containing ART.35 Patients were randomized to receive their maintenance dose of darunavir (800 mg) once daily, or to switch to a lower dose (600 mg) twice daily during a pretreatment period. Regardless of the dose, 100% of the patients achieved SVR12.
EBR/GZR was studied in HIV/HCV-coinfected patients in the phase 3, open-label, single-arm C-EDGE COINFECTION study.36 Efficacy, safety, and tolerability were assessed in 218 patients given once-daily oral administration of 50 mg EBR plus 100 mg GZR in a fixed-dose combination tablet for 12 weeks. All patients had not received treatment for HCV, had GT1 or 4, and were ART-naive or stable on their current regimen for at least 8 weeks. Overall SVR12 was 98%, and 100% for the 35 patients with cirrhosis.
SVR12 results from the ASTRAL-5 trial were recently reported.37 In this single-arm, phase 3 study in patients with HCV GT 1, 2, 3, or 4 coinfected with HIV, 95% (101/106) achieved SVR12 with 12 weeks of SOF/VEL.
HIV/HCV-coinfected patients are no longer considered a “hard-to-cure” patient population since the high SVR12 rates are comparable between monoinfected and coinfected patients. However, evidence suggests that the incidence of HCV reinfection post-SVR may be higher in patients with HIV infection. In a meta-analysis of 66 studies with 11,071 patients who achieved SVR with interferon/ribavirin regimens, the pooled recurrence rate in “low-risk” HCV monoinfected patients was 1.85/1000 person-years of follow-up (PYFU), indicating a 5-year recurrence risk of 0.95%.38 The pooled recurrence rate for “high-risk” (intravenous drug users/prisoners) HCV-monoinfected patients was much higher (22.32/1000 PYFU) and associated with a 5-year risk of 10.67%. HIV/HCV-coinfected patients showed the highest pooled recurrence rate (32.02/1000 PYFU) and 5-year risk (15.02%). Recurrence is driven not by late relapse, but rather by increased reinfection. A recent report demonstrated that an HIV-infected man with a telaprevir-resistant HCV (V36M) RAV transmitted the resistant virus to a male sex partner. This scenario presents a clinical challenge, as transmission of RAVs could act as a potential confounder in DAA-containing therapeutic regimens.
Guidance from the AASLD/IDSA recommends that “HIV/HCV-co-infected persons should be treated and re-treated the same as persons without HIV infection, after recognizing and managing interactions with antiretroviral medications.”1 However, many experts would not recommend the widely used 8-week LDV/SOF regimen for coinfection in the following setting: noncirrhotic patients with GT1 who had not yet received treatment for HCV and who have an HCV RNA of less than 6 million IU/mL.
Regarding the potential for drug-drug interactions, recommendations from the AASLD/IDSA provide clear guidance regarding which combinations are considered safe.1 When changes in a patient’s ART regimen are necessary because of anticipated HCV therapy, they recommend substitution of an integrase inhibitor for a protease inhibitor. This can nearly always be accomplished without risking recrudescent HIV replication, although patients may require appropriate reassurances. Many physicians who treat HCV will feel it advisable to consult the patient’s HIV physician about such changes to treatment, unless they customarily manage HIV infection themselves.
Renal Insufficiency
The estimated prevalence of HCV infection in patients with end-stage renal disease (ESRD) in the United States is about 8%, with approximately 400,000 patients on hemodialysis. This prevalence rate is roughly 5 times greater than that observed for the general population in the United States. In hemodialysis patients, a history of HCV infection is independently associated with increased mortality, as well as increased rates of cirrhosis and hepatocellular carcinoma.39
Before the introduction of the currently used DAA regimens, HCV-infected patients with renal insufficiency/ESRD had major limitations concerning treatment. Interferon and ribavirin caused significant complications, especially anemia, in patients with renal disease. The risk of anemia required initial or on-treatment ribavirin dose reductions, and tolerability issues led to poor adherence and SVR12 rates. In addition, treatment posttransplant was limited because of the high risk for graft rejection associated with interferon and ribavirin-associated anemia.
Several studies have been conducted to assess the efficacy and safety of DAA regimens in HCV-infected patients with severe renal impairment (defined by chronic kidney disease stages 4 and 5 or need for hemodialysis). In the RUBY-1 trial, 20 treatment-naive HCV GT1a and 1b patients were given PTV/r/OBV plus DSV for 12 weeks.40 The 13 patients with GT1a infections also received RBV. As a result of underlying renal dysfunction, the RBV dose was 200 mg/d for GT1a patients, with provisions to discontinue if hemoglobin levels declined by more than 2 g/dL. Although frequent hemoglobin declines were observed, they were managed with RBV interruption and erythropoietin administration as needed and did not have a detectable effect on overall treatment efficacy. The overall SVR12 was 90%.
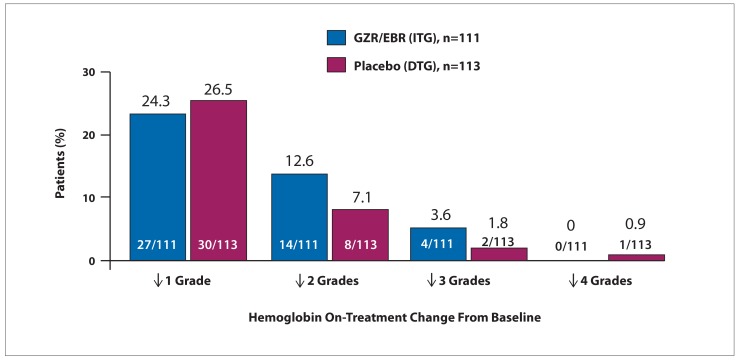
The phase 3 C-SURFER study assessed the efficacy and safety of GZR/EBR in HCV treatment-naive and treatment-experienced patients with GT1 infection and severe renal impairment.41 Patients with compensated cirrhosis were also included, and 75% of patients were on dialysis. A total of 224 patients were randomized to immediate treatment with GZR/EBR or deferred treatment, with patients who received placebo for 12 weeks treated subsequently with open-label GZR/EBR. In a modified intent-to-treat analysis that excluded patients with nonvirologic failure, SVR12 was achieved by 99% of patients who completed treatment. Six patients discontinued early for reasons unrelated to treatment. As ribavirin was not part of the regimen, hemoglobin levels were not impacted by treatment. The on-treatment changes in hemoglobin are shown in Figure 3. This study may be regarded as important because of the unmet need it addressed in this difficult-to-treat population.
Figure 3.
On-treatment changes in hemoglobin from baseline in HCV-infected patients with severe renal impairment treated with grazoprevir/elbasvir.
DTG, deferred-treatment group; GZR/EBR, grazoprevir/elbasvir; HCV, hepatitis C virus; ITG, immediate-treatment group. Adapted from Roth D et al. Lancet. 2015;386(10003):1537-1545.41
Unique among the available DAAs, SOF has a predominantly renal excretory pathway. Its major metabolite accumulates up to 20-fold in patients with renal failure, leading to potential safety concerns. An early trial studied SOF (200 mg) plus RBV in patients with severe renal impairment.42,43 The combination was safe and relatively well-tolerated in patients with severe renal impairment. Exacerbation of anemia via RBV-induced hemolysis was the primary adverse event. In addition, an observational study (HCV TARGET) assessed the rate of SVR12 associated with SOF regimens by baseline estimated glomerular filtration rate (eGFR).44 SOF/SMV was the most common treatment regimen used, and SVR12 was similar (>80%) regardless of baseline eGFR. Rates of anemia, worsening renal function, and renal and urinary adverse events were higher in patients with lower eGFR and warrant the need for close monitoring in these patients.
In summary, the AASLD/IDSA guidance document recommends GZR/EBR as a safe and highly effective treatment in GT1a, 1b, and 4 patients with severe renal impairment, including those on hemodialysis. It should be noted that the package insert for GZR/EBR does not exempt patients with renal failure from the stipulation that GT1a patients should have baseline RAV testing and, if present, receive the regimen with ribavirin for 16 weeks. Nevertheless, the high SVR rates in C-SURFER have raised the possibility that baseline RAVs may not impact SVR in GT1a patients, for unclear reasons. More data are needed, but many clinicians choose not to use ribavirin in this population. The guidance document also recommends using PTV/r/OBV plus DSV without ribavirin in GT1b patients with advanced renal disease. A current study is evaluating whether GT1a patients can receive this regimen effectively without the addition of ribavirin. Safety data for SOF-containing regimens in renal impairment are awaited, although real-world data have indicated high efficacy in small studies with either a full dose of sofosbuvir or dosing every other day.44,45 Presently, the use of sofosbuvir in patients with severe renal impairment is neither FDA-approved nor recommended by the AASLD/IDSA guidance document pending establishment of an appropriate dose.
Biography

Footnotes
Funding for this supplement has been provided by Bristol-Myers Squibb and Merck & Co. Support of this supplement does not imply the supporter’s agreement with the views expressed herein. Every effort has been made to ensure that drug usage and other information are presented accurately; however, the ultimate responsibility rests with the prescribing physician. Gastro-Hep Communications, Inc., the supporter, and the participants shall not be held responsible for errors or for any consequences arising from the use of information contained herein. Readers are strongly urged to consult any relevant primary literature. No claims or endorsements are made for any drug or compound at present under clinical investigation.
Disclosure of Conflicts of Interest: All faculty and staff involved in the planning or presentation of continuing education activities sponsored/provided by the Annenberg Center for Health Sciences at Eisenhower (ACHS) are required to disclose to the audience any real or apparent commercial financial affiliations related to the content of the presentation or enduring material. Full disclosure of all commercial relationships must be made in writing to the audience prior to the activity. John Bayliss, VP, Business Development, spouse is an employee of Amgen, Inc.; all other staff at the Annenberg Center for Health Sciences at Eisenhower and the Chronic Liver Disease Foundation (CLDF) have no relationships to disclose.
The opinions expressed in this supplement are those of the faculty and do not necessarily represent the views of the Annenberg Center for Health Sciences at Eisenhower. The information is presented for the purpose of advancing the readers’ professional development.
Disclosure: Dr Jacobson has received grant/research support from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck. He is on the Speakers Bureaus of AbbVie, Bristol-Myers Squibb, Gilead, and Janssen. He is a consultant/advisory board member of AbbVie, Achillion, Bristol-Myers Squibb, Intercept, Gilead, Janssen, Merck, and Trek.
References
- 1.HCV guidance: recommendations for testing, managing, and treating hepatitis C. American Association for the Study of Liver Diseases/Infectious Diseases Society of America. [Accessed September 16, 2016]. http://www.hcvguidelines.org Updated September 16, 2016.
- 2.Afdhal N, Zeuzem S, Kwo P, et al. ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 3.Afdhal N, Reddy KR, Nelson DR, et al. ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 4.Kowdley KV, Gordon SC, Reddy KR, et al. ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 5.Zeuzem S, Mizokami M, Pianko S, et al. Prevalence of pre-treatment NS5A resistance associated variants in genotype 1 patients across different regions using deep sequencing and effect on treatment outcome with LDV/SOF [AASLD Meeting Abstract 91] Hepatology. 2015;62(suppl 1) [Google Scholar]
- 6.Sarrazin C, Dvory-Sobol H, Svarovskaia ES, et al. Prevalence of resistance-associated substitutions in HCV NS5A, NS5B, or NS3 and outcomes of treatment with ledipasvir and sofosbuvir. Gastroenterology. 2016;151(3):501–512. doi: 10.1053/j.gastro.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Feld JJ, Jacobson IM, Hézode C, et al. ASTRAL-1 Investigators. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 8.Gane EJ, Shiffman ML, Etzkorn K. Sofosbuvir/velpatasvir in combination with ribavirin for 24 weeks is effective retreatment of patients who failed prior NS5A containing DAA regimens: results of the GS-US-342-1553 study [EASL abstract PS024] J Hepatol. 2016;64(suppl 2) [Google Scholar]
- 9.Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevirelbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med. 2015;163(1):1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 10.Kwo P, Gane E, Peng C-Y, et al. Efficacy and safety of grazoprevir/elbasvir +/- RBV for 12 weeks in patients with HCV G1 or G4 infection who previously failed peginterferon/RBV: C-edge treatment-experienced trial [EASL abstract P0886] J Hepatol. 2015;62(suppl 2) [Google Scholar]
- 11.Poordad F, Gordon SC, Asatryan A, et al. High-efficacy of ABT-493 and ABT-530 in HCV genotype-1-infected patients who have failed direct-acting antiviral-containing regimens: the Magellan-1 study [EASL abstract GS11] J Hepatol. 2016;64(suppl 2) [Google Scholar]
- 12.Lawitz E, Kowdley K, Curry M, et al. High efficacy of sofosbuvir/velpatasvir plus GS-9857 for 12 weeks in treatment-experienced genotype 1-6 HCV-infected patients, including those previously treated with direct-acting antivirals [EASL abstract PS008] J Hepatol. 2016;64(suppl 2) [Google Scholar]
- 13.Gane EJ, Gao W, Huang H, et al. Phase 2, randomized, open-label clinical trials of the efficacy and safety of grazoprevir and MK-3682 (NS5B polymerase inhibitor) with either elbasvir or MK-8408 (NS5A inhibitor) in patients with chronic HCV GT1, 2 or 3 infection (part A of C-CREST-1 & 2) [AASLD Meeting Abstract LB-15] Hepatology. 2015;62(suppl 1) [Google Scholar]
- 14.Foster GR, Afdhal N, Roberts SK, et al. ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373(27):2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 15.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 16.Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. veterans with HCV. Hepatology. 2014;60(1):98–105. doi: 10.1002/hep.27095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson DR, Cooper JN, Lalezari JP, et al. ALLY-3 Study Team. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61(4):1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leroy V, Angus P, Bronowicki JP, et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY-3+) Hepatology. 2016;63(5):1430–1441. doi: 10.1002/hep.28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asselah T, Reesink HW, Gerstoft J, et al. High efficacy of elbasvir and grazoprevir with or without ribavirin in 103 treatment-naive and experienced patients with HCV genotype 4 infection: a pooled analysis [AASLD Meeting Abstract 251] Hepatology. 2015;62(suppl 1) [Google Scholar]
- 20.Kohli A, Kapoor R, Sims Z, et al. Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis. 2015;15(9):1049–1054. doi: 10.1016/S1473-3099(15)00157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hézode C, Asselah T, Reddy KR, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet. 2015;385(9986):2502–2509. doi: 10.1016/S0140-6736(15)60159-3. [DOI] [PubMed] [Google Scholar]
- 22.Abergel A, Asselah T, Metivier S, et al. Ledipasvirsofosbuvir in patients with hepatitis C virus genotype 5 infection: an open-label, multicentre, single-arm, phase 2 study. Lancet Infect Dis. 2016;16(4):459–464. doi: 10.1016/S1473-3099(15)00529-0. [DOI] [PubMed] [Google Scholar]
- 23.Epclusa [package insert]. Foster City, CA: Gilead Sciences. 2016.
- 24.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34(6):831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 25.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected person. Ann Intern Med. 2003;138(3):197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- 26.Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS. 2015;29(17):2335–2345. doi: 10.1097/QAD.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramachandran S, Teshale E, Switzer W, et al. Networks of HCV transmissions among persons who inject drugs: Indiana, 2015. Paper presented at: 2016 Conference on Retroviruses and Opportunistic Infections; February 22-25, 2016; Boston, Massachusetts. Abstract 149. [Google Scholar]
- 28.Smith CJ, Ryom L, Weber R, et al. D:A:D Study Group. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 29.Lo Re V, III, Kallan MJ, Tate JP, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med. 2014;160(6):369–379. doi: 10.7326/M13-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Althoff KN, Estrella M, Abraham A, et al. The racial survival paradox in HIV+ end-stage renal disease (ESRD) patients. Presented at: 2016 Conference on Retroviruses and Opportunistic Infections; February 22-25, 2016; Boston, Massachusetts. Abstract 688. [Google Scholar]
- 31.Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-term treatment outcomes of patients infected with hepatitis C virus: a systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clin Infect Dis. 2015;61(5):730–740. doi: 10.1093/cid/civ396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyles DL, Ruane PJ, Sulkowski MS, et al. ALLY-2 Investigators. Daclatasvir plus sofosbuvir for HCV in patients co-infected with HIV-1. N Engl J Med. 2015;373(8):714–725. doi: 10.1056/NEJMoa1503153. [DOI] [PubMed] [Google Scholar]
- 33.Naggie S, Cooper C, Saag M, et al. ION-4 Investigators. Ledipasvir and sofosbuvir for HCV in patients co-infected with HIV-1. N Engl J Med. 2015;373(8):705–713. doi: 10.1056/NEJMoa1501315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sulkowski MS, Eron JJ, Wyles D, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015;313(12):1223–1231. doi: 10.1001/jama.2015.1328. [DOI] [PubMed] [Google Scholar]
- 35.Wyles D, Saag M, Trinh R, et al. TURQUOISE-I Part 1b: ombitasvir/paritaprevir/r+dasabuvir+RBV for HCV/HIV coinfection. Presented at: 2016 Conference on Retroviruses and Opportunistic Infections; February 22-25, 2016; Boston, Massachusetts. Abstract 574. [Google Scholar]
- 36.Rockstroh JK, Nelson M, Katlama C, et al. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV. 2015;2(8):e319–e327. doi: 10.1016/S2352-3018(15)00114-9. [DOI] [PubMed] [Google Scholar]
- 37.Wyles D, Brau N, Kottilil S, et al. Sofosbuvir/velpatasvir for 12 weeks in patients coinfected with HCV and HIV-1: the ASTRAL-5 study [EASL abstract PS104] J Hepatol. 2016;64(suppl 2) [Google Scholar]
- 38.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis. 2016;62(6):683–694. doi: 10.1093/cid/civ948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kidney disease statistics for the United States. National Institute of Diabetes and Digestive and Kidney Diseases. [Accessed July 18, 2016]. http://kidney.niddk.nih.gov/kudiseases/pubs/kustats/ Updated September 2015.
- 40.Pockros PJ, Reddy KR, Mantry PS, et al. Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology. 2016;150(7):1590–1598. doi: 10.1053/j.gastro.2016.02.078. [DOI] [PubMed] [Google Scholar]
- 41.Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386(10003):1537–1545. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 42.Cornpropst MT, Denning JM, Clemons D, et al. The effect of renal impairment and end stage renal disease on the single-dose pharmacokinetics of PSI-7977 [EASL abstract 1101] J Hepatol. 2012;56:S433. [Google Scholar]
- 43.Gane EJ, Robson RA, Bonacini M, et al. Safety, antiviral efficacy and pharmacokinetics (PK) of sofosbuvir (SOF) in patients with severe renal impairment [AASLD Meeting Abstract 966] Hepatology. 2015;60(suppl 1) [Google Scholar]
- 44.Saxena V, Koraishy FM, Sise ME, et al. HCV-TARGET. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int. 2016;36(6):807–816. doi: 10.1111/liv.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nazario HE, Ndungu M, Modi AA. Sofosbuvir and simeprevir in hepatitis C genotype 1-patients with end-stage renal disease on haemodialysis or GFR <30 ml/min. Liver Int. 2016;36(6):798–801. doi: 10.1111/liv.13025. [DOI] [PubMed] [Google Scholar]