Abstract Abstract
Studies have suggested roles for angiopoietin-2 (Ang-2) and soluble P-selectin (sP-selectin) as biomarkers of disease severity and treatment response in pulmonary arterial hypertension (PAH), but additional data are required for validation. We evaluated these biomarkers using data from FREEDOM-C2, in which patients with PAH receiving stable monotherapy or combination therapy were randomized to receive additional treatment with oral treprostinil (up-titrated from 0.25 mg twice daily) or placebo for 16 weeks. Biomarker analysis was optional in FREEDOM-C2. We measured plasma Ang-2 and sP-selectin levels at baseline and at week 16, and we assessed their association with predefined outcomes (6-minute walk distance [6MWD] change from baseline >40 m, 6MWD >380 m, functional class I/II, and/or N-terminal pro-brain natriuretic peptide [NT-proBNP] <1,800 pg/mL at week 16) using Spearman correlation, receiver operating characteristics, and logistic regression. Biomarker data were available for 83 of 157 and 95 of 153 patients in the oral treprostinil and placebo groups, respectively. In the oral treprostinil group, baseline Ang-2 levels correlated with week 16 NT-proBNP levels (P < 0.0001). Baseline Ang-2 ≥12 ng/mL was associated with a reduced likelihood of having NT-proBNP <1,800 pg/mL at week 16 (multivariate odds ratio: 0.08; 95% confidence interval: 0.02–0.32). However, Ang-2 showed no significant association with the other assessed outcomes, and sP-selectin was not associated or correlated with any of the outcomes. These data suggest that Ang-2 and sP-selectin are not associated with response to oral treprostinil in patients already receiving stable PAH therapy. Trial registration: Clinicaltrials.gov identifier NCT00887978.
Keywords: angiopoietin-2, granule membrane protein 140, prostacyclin, pulmonary hypertension
The role of biomarkers is to support clinical decision-making and patient education with respect to diagnosis, prognosis, and therapeutic intervention. Advances in the understanding of the pathophysiology and pathogenesis of pulmonary arterial hypertension (PAH) have resulted in the identification of several potential biomarkers, including markers of myocyte insult (e.g., brain natriuretic peptide [BNP] and N-terminal pro-BNP [NT-proBNP]), endothelial dysfunction/remodeling (e.g., endothelin-1), inflammation or oxidative stress (e.g., C-reactive protein), and end-organ dysfunction (e.g., creatinine).1 BNP and NT-proBNP have been adopted as prognostic biomarkers in PAH management guidelines, but they tend to have high variability and are not specific for pulmonary hypertension.2,3 The search for additional potential biomarkers therefore continues, with recently identified candidates including adiponectin, angiopoietin-2 (Ang-2), cystatin C, growth differentiation factor 15, intercellular adhesion molecule 1, osteopontin, platelet endothelial cell adhesion molecule, P-selectin, and tenascin-C.1,4-7
Ang-2 has shown very promising results as a biomarker: results from a cohort study involving patients with idiopathic PAH suggested that it could be a better predictor of survival than the established biomarker, NT-proBNP.8 Ang-2 is stored in secretory organelles, known as Weibel–Palade bodies, in endothelial cells; it is released in response to various stimuli that include thrombin and histamine, and its expression is increased in hypoxic conditions.9-11 Ang-2 binds the endothelial receptor Tie-2 and antagonizes Ang-1, thereby causing a destabilization of the endothelium.12,13 In the presence of vascular endothelial growth factor, this leads to angiogenesis, whereas in the absence of vascular endothelial growth factor, it leads to endothelial cell death and vessel regression.14 Elevated levels of Ang-2 have been reported in patients with chronic and acute heart failure15-17 as well as in patients with PAH.7,8 In a cohort study involving 106 patients with idiopathic PAH, changes of Ang-2 during follow-up were closely related to treatment response, and Ang-2 correlated with disease severity and was an independent predictor of outcome.8 In a randomized, controlled trial in a group of patients with PAH, Ang-2 was decreased from baseline in 12 of 13 patients with available biomarker data after 3 months of treatment with intravenous treprostinil.7
Ang-2 has been shown to induce cell surface translocation of P-selectin, which is also a potential PAH biomarker.6,18,19 P-selectin is a transmembrane glycoprotein that occurs in endothelial Weibel-Palade bodies and in platelets.20 When transported to the endothelial cell surface, P-selectin mediates leukocyte adhesion and rolling.21 A soluble form of P-selectin (sP-selectin) exists in plasma and is thought to reflect platelet activation and/or endothelial cell injury.22,23 Increased levels of sP-selectin were found in patients with PAH compared with healthy controls,23-26 and administration of intravenous prostacyclin to 18 patients with PAH led to a reduction in sP-selectin levels from baseline after a mean follow-up period of 90 days, suggesting an improvement in endothelial injury and altered hemostasis.23
Ang-2 and sP-selectin have not yet been extensively investigated in a clinical setting, and data on their response to specific PAH monotherapy or combination therapy are limited; additional data are needed to validate their potential role as biomarkers. We therefore assessed Ang-2 and sP-selectin levels in patients with PAH participating in the FREEDOM-C2 study of oral treprostinil.27 We hypothesized that Ang-2 and sP-selectin levels are associated with the treatment response when oral treprostinil is administered in combination with other PAH medications and that they may therefore have an impact on clinical decision-making and disease monitoring.
Methods
Study design and patients
Details of the study design have been described elsewhere.27 Briefly, FREEDOM-C2 was an international, randomized, double-blind, placebo-controlled, 16-week trial. Adult patients with PAH who were already being treated with an endothelin receptor antagonist and/or a phosphodiesterase 5 inhibitor (for ≥90 days before baseline and at a stable dose for ≥30 days before baseline) were randomized 1∶1 to receive oral treprostinil or placebo on top of their existing treatment. Randomization was stratified by background therapy and 6-minute walk distance (6MWD; ≤350 m or >350 m). The starting dosage of the study drug was 0.25 mg twice daily, and the dose could be titrated upward every 3 days if clinically indicated (in increments of 0.25 mg in the first 4 weeks and 0.25 mg or 0.5 mg thereafter). The study design included assessment of outcomes such as 6MWD, World Health Organization (WHO)/New York Heart Association (NYHA) functional class, and NT-proBNP levels as well as optional blood sampling for biomarker analysis. It did not include invasive hemodynamic or echocardiographic assessments. The FREEDOM-C2 protocol was approved by institutional review boards at all participating study centers, and all patients provided written informed consent.27 The current post hoc analysis was conducted in the subset of patients who provided optional blood samples for biomarker analysis and who had Ang-2 and sP-selectin data available both at baseline and at week 16.
Study objectives
The primary objectives of the current substudy were to compare Ang-2 and sP-selectin levels at baseline and week 16 and their change from baseline to week 16 in responders and nonresponders to treprostinil therapy. Ang-2 and sP-selectin were assessed in EDTA plasma using a multiplexed immunoassay (DiscoveryMAP ver. 3.0 assay; Myriad RBM, Austin, Texas). Treatment response was predefined in five different ways for the current study: (1) 6MWD change from baseline at week 16 >40 m (adapted from two previous studies that identified 38.6 m and 41.8 m as significant thresholds),28,29 (2) 6MWD >380 m at week 16 (as assessed by Hoeper et al.30 and recommended by McLaughlin et al.31 for goal-directed therapy), (3) WHO/NYHA functional class I/II at week 16 (based on European guidelines2 and the recommendations of McLaughlin et al.31), (4) NT-proBNP <1,800 pg/mL at week 16 (based on the suggestions for treatment goals made by McLaughlin et al.31), and (5) all of the previous four criteria (hereafter referred to as overall response).
The secondary objectives were to compare Ang-2 and sP-selectin at baseline and at week 16 and the change in Ang-2 and sP-selectin from baseline to week 16 between low-dose and high-dose treprostinil groups (≤3.1 mg and >3.1 mg twice daily at week 16, respectively). The dose threshold was the mean dose of oral treprostinil received by patients at week 16 (3.1 ± 1.9 mg twice daily).27
Statistical analysis
Data are expressed as mean ± standard deviation (SD), median (range), or number (%) for normally distributed parameters, nonnormally distributed parameters, or categorical parameters, respectively. Between-group differences were analyzed with a two-sample t test for normally distributed parameters, the Mann-Whitney U test for nonnormally distributed parameters, and the Fisher exact test for categorical parameters. P < 0.05 was considered statistically significant for comparison between groups. Analyses of the relationship of Ang-2 and sP-selectin levels with treatment response were conducted in the oral treprostinil group only. The correlation of Ang-2 and sP-selectin with 6MWD, WHO/NYHA functional class, and NT-proBNP was assessed in the oral treprostinil group using Spearman’s methods. Receiver operating characteristic (ROC) analyses were performed to evaluate the potential utility of Ang-2 and sP-selectin thresholds as predictors of treatment response. Cutoff points were determined using the Youden index. Univariate and multivariate logistic regression analyses were used to clarify whether baseline Ang-2 or sP-selectin levels (dichotomized at the median or the threshold value obtained from ROC analysis if the latter had sufficient power [area under the ROC curve >0.8]) were independently associated with therapy response (based on individual response criteria as well as overall response). Baseline 6MWD, age, sex, WHO/NYHA functional class, Borg index, and disease etiology were included in the multivariate model.
Results
Baseline characteristics
Of the 310 patients in the FREEDOM-C2 study, 157 were randomized to receive oral treprostinil, and 153 were randomized to receive placebo. Baseline characteristics of the randomized patients are presented in Table 1. There were no significant differences in baseline characteristics between the oral treprostinil group and the placebo group. All included patients were receiving stable background PAH therapy (as stipulated by the study protocol), and many of the patients were already receiving dual combination therapy with both an endothelin receptor antagonist and a phosphodiesterase 5 inhibitor at baseline (41.4% of the oral treprostinil group and 39.2% of the placebo group). Of the randomized patients, 209 provided blood samples for biomarker analysis, and 178 (83 in the oral treprostinil group and 95 in the placebo group) had biomarker data both at baseline and at week 16. Baseline characteristics showed no significant differences between patients with and patients without biomarker data (Table 2).
Table 1.
Baseline characteristics of randomized patients
| Variable | Oral treprostinil (N = 157) |
Placebo (N = 153) |
P value |
|---|---|---|---|
| Age, years | 52.0 (18.0–76.0) | 50.0 (20.0–75.0) | 0.470a |
| Female sex | 119 (75.8) | 122 (79.7) | 0.404 |
| PAH etiology: | 0.661b | ||
| Idiopathic or familial | 104 (66.2) | 99 (64.7) | |
| Connective tissue disease | 48 (30.6) | 49 (32.0) | |
| HIV infection | 2 (1.3) | 4 (2.6) | |
| Congenital heart disease | 3 (1.9) | 1 (.7) | |
| NYHA/WHO functional class:c | 0.217b | ||
| II | 43 (27.6) | 37 (24.3) | |
| III | 110 (70.5) | 115 (75.7) | |
| IV | 3 (1.9) | 0 (.0) | |
| 6MWD, m | 359.0 (150.0–422.0) | 355.0 (152.0–422.0) | 0.386a |
| NT-proBNP, pg/mLd | 712.0 (25.5–11,256.0) | 796.0 (25.5–20,456.0) | 0.392a |
| Background PAH therapy: | 0.840 | ||
| ERA | 25 (15.9) | 28 (18.3) | |
| PDE5I | 67 (42.7) | 65 (42.5) | |
| ERA + PDE5I | 65 (41.4) | 60 (39.2) | |
| Years since PAH diagnosis | 1.7 (0.0–15.0) | 1.9 (0.0–26.4) | 0.367a |
| Angiopoietin-2, ng/mLe | 9.3 (1.8–52.0) | 8.4 (2.5–43.0) | 0.638a |
| Soluble P-selectin, ng/mLe | 64.0 (21.0–146.0) | 67.0 (33.0–800.0) | 0.234a |
Adapted from Tapson et al.25 Data are presented as median (range) or number (%). 6MWD: 6-minute walk distance; ERA: endothelin receptor antagonist; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; PAH: pulmonary arterial hypertension; PDE5I: phosphodiesterase 5 inhibitor; WHO: World Health Organization.
Nonparametric test (Mann-Whitney U test).
Fisher exact test.
Data available for 156 and 152 patients in the oral treprostinil and placebo groups, respectively.
Data available for 149 and 148 patients in the oral treprostinil and placebo groups, respectively.
Data available for 83 and 95 patients in the oral treprostinil and placebo groups, respectively.
Table 2.
Baseline characteristics of patients with and patients without biomarker data at baseline and week 16
| Variable | Patients with biomarker data (N = 178) |
Patients without biomarker data (N = 132) |
P value |
|---|---|---|---|
| Age, years | 49.5 (20.0–76.0) | 53.0 (18.0–75.0) | 0.437a |
| Female sex | 136 (76.4) | 105 (79.5) | 0.511 |
| PAH etiology: | 0.400b | ||
| Idiopathic or familial | 110 (61.8) | 93 (70.5) | |
| Connective tissue disease | 62 (34.8) | 35 (26.5) | |
| HIV infection | 4 (2.2) | 2 (1.5) | |
| Congenital heart disease | 2 (1.1) | 2 (1.5) | |
| NYHA/WHO functional class:c | 0.252b | ||
| II | 41 (23.2) | 39 (29.8) | |
| III | 135 (76.3) | 90 (68.7) | |
| IV | 1 (.6) | 2 (1.5) | |
| 6MWD, m | 360.0 (150.0–422.0) | 352.0 (156.0–422.0) | 0.590a |
| NT-proBNP, pg/mLd | 779.7 (25.5–20,456.0) | 659.0 (25.5–10,360.0) | 0.666a |
| Background PAH therapy: | 0.541 | ||
| ERA | 27 (15.2) | 26 (19.7) | |
| PDE5I | 79 (44.4) | 53 (40.2) | |
| ERA + PDE5I | 72 (40.4) | 53 (40.2) | |
| Years since PAH diagnosis | 1.7 (0.0–24.0) | 1.8 (0.0–26.4) | 0.440a |
Data are presented as median (range) or number (%). 6MWD: 6-minute walk distance; ERA: endothelin receptor antagonist; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; PAH: pulmonary arterial hypertension; PDE5I: phosphodiesterase 5 inhibitor; WHO: World Health Organization.
Nonparametric test (Mann-Whitney U test).
Fisher exact test.
Data available for 177 and 131 patients in the groups with and without biomarker data, respectively.
Data available for 174 and 123 patients in the groups with and without biomarker data, respectively.
Effect of oral treprostinil on Ang-2 and sP-selectin levels
At week 16, no significant differences in Ang-2 or sP-selectin levels were observed between the oral treprostinil and placebo groups. The median level of Ang-2 in the oral treprostinil group was 8.4 mg/mL, compared with 7.8 ng/mL in the placebo group (P = 0.775). The corresponding median levels of sP-selectin were 66.0 ng/mL and 65.0 ng/mL, respectively (P = 0.375). The change in Ang-2 or sP-selectin from baseline was also not significantly different between the two treatment groups; Ang-2 showed a slight difference that approached statistical significance but was not considered to be clinically relevant (median: −0.2 ng/mL [oral treprostinil] vs. +0.2 ng/mL [placebo]; P = 0.064), whereas sP-selectin showed no difference (median: 0.0 ng/mL [oral treprostinil] vs. 2.0 ng/mL [placebo]; P = 0.752).
Association of Ang-2 and sP-selectin levels with treatment response at week 16
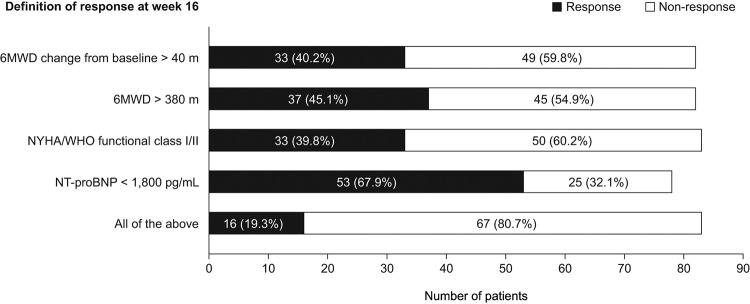
The proportions of patients in the oral treprostinil group who showed a treatment response at week 16 (based on individual response criteria and overall response) are displayed in Figure 1. Ang-2 and sP-selectin levels at baseline and at week 16 and their change from baseline to week 16 were compared between treatment response and nonresponse subgroups. Ang-2 levels at baseline and at week 16 were both significantly lower in patients with versus patients without a treatment response, defined as NT-proBNP <1,800 pg/mL (median: 7.6 vs. 16.0 ng/mL [baseline] and 6.3 vs. 17.0 ng/mL [week 16]; both P < 0.001). The change from baseline to week 16 in sP-selectin level was significantly greater in patients whose 6MWD improved >40 m from baseline compared with those who did not show such an improvement (median: +8.0 vs. −1.0 ng/mL; P = 0.041) and in patients who showed an overall treatment response at week 16 compared with those who did not show an overall response (median: +10.5 vs. −1.0 ng/mL; P = 0.043). No other significant differences in Ang-2 and sP-selectin levels were found between response subgroups. The median change from baseline to week 16 in Ang-2 level was −1.0 versus 0.0 ng/mL (P = 0.249) in patients subdivided by change from baseline in 6MWD (>40 vs. ≤40 m), −0.2 versus −0.3 ng/mL (P = 0.978) in patients subdivided by absolute 6MWD at week 16 (>380 vs. ≤380 m), −0.7 versus 0.0 ng/mL (P = 0.402) in patients subdivided by WHO/NYHA functional class at week 16 (I/II vs. III/IV), −0.2 versus −0.6 ng/mL (P = 0.835) in patients subdivided by NT-proBNP level at week 16 (<1,800 vs. ≥1,800 pg/mL), and −0.1 versus −0.3 ng/mL (P = 0.804) in patients subdivided by overall response (responder vs. nonresponder). The median change from baseline to week 16 in sP-selectin level was 2.0 versus −1.0 ng/mL (P = 0.505) in patients subdivided by absolute 6MWD at week 16 (>380 vs. ≤380 m), 3.0 versus −1.0 ng/mL (P = 0.280) in patients subdivided by WHO/NYHA functional class at week 16 (I/II vs. III/IV), and 3.0 versus 1.0 ng/mL (P = 0.889) in patients subdivided by NT-proBNP level at week 16 (<1,800 vs. ≥1,800 pg/mL).
Figure 1.
Treatment response at week 16 for patients receiving oral treprostinil (subset with available data on angiopoietin-2 and soluble P-selectin; n = 83). 6MWD: 6-minute walk distance; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; WHO: World Health Organization.
The correlation of individual treatment response parameters with Ang-2 and sP-selectin levels is summarized in Table 3. One significant correlation was found: baseline Ang-2 levels correlated with NT-proBNP levels at week 16 (P < 0.0001; Table 3 and Fig. 2).
Table 3.
Spearman correlation coefficients and P values for association of angiopoietin-2 (Ang-2) and soluble P-selectin (sP-selectin) levels with clinical parameters at week 16 in patients receiving oral treprostinil (n = 83)
| Correlation coefficient (P value) | 6MWD at week 16, m | NYHA/WHO functional class at week 16 | NT-proBNP at week 16, pg/mL | Oral treprostinil dose at week 16, mg BID |
|---|---|---|---|---|
| Ang-2 at baseline, ng/mL | −0.19 (0.0906) | 0.19 (0.0940) | 0.59 (<0.0001a) | −0.18 (0.1148) |
| sP-selectin at baseline, ng/mL | −0.15 (0.1747) | 0.07 (0.5279) | 0.08 (0.4899) | 0.06 (0.5831) |
| Ang-2 change from baseline, ng/mL | −0.05 (0.6530) | 0.10 (0.3481) | 0.06 (0.5995) | −0.00 (1.0000) |
| sP-selectin change from baseline, ng/mL | 0.02 (0.8646) | −0.09 (0.4076) | −0.01 (0.9131) | −0.06 (0.6092) |
6MWD: 6-minute walk distance; BID: twice daily; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; WHO: World Health Organization.
Significant at type I error level of 0.05.
Figure 2.
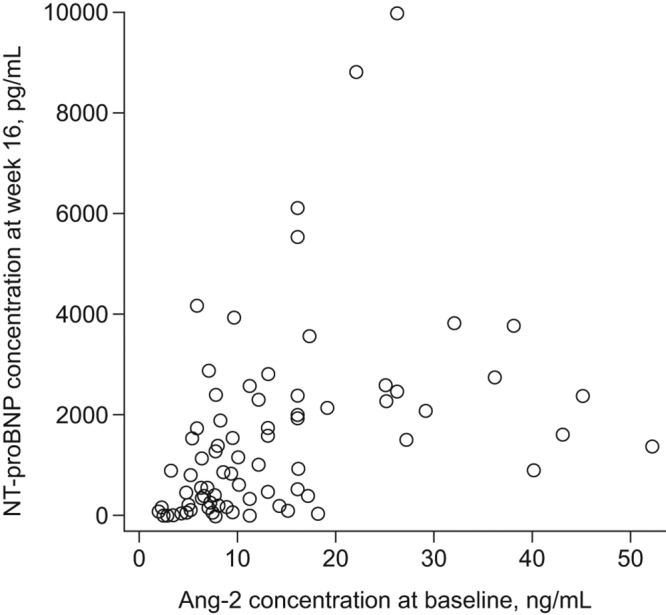
Scatter plot showing the relationship between Ang-2 concentration at baseline and NT-proBNP concentration at week 16 for patients receiving oral treprostinil (subset with available data on Ang-2 and NT-proBNP; n = 78). Ang-2: angiopoietin-2; NT-proBNP: N-terminal pro-brain natriuretic peptide.
The results of ROC analyses to assess the potential of baseline Ang-2 and sP-selectin levels as predictors of response to oral treprostinil therapy (based on individual response criteria and overall response) are displayed in Table 4. Most of the results were around 0.5, indicating insufficient power to use the biomarker thresholds obtained from the ROC analyses to predict treatment response. The exception was baseline Ang-2 level as a predictor of NT-proBNP <1,800 pg/mL, for which an ROC threshold of 12 ng/mL was identified.
Table 4.
Receiver-operating characteristic (ROC) analyses of baseline angiopoietin-2 (Ang-2) and soluble P-selectin (sP-selectin) levels as potential predictors of response to oral treprostinil therapy at week 16
| Area under the ROC curve | ||
|---|---|---|
| Definition of response at week 16 | Ang-2 at baseline | sP-selectin at baseline |
| 6MWD change from baseline >40 m | 0.566 | 0.560 |
| 6MWD >380 m | 0.592 | 0.573 |
| NYHA/WHO functional class I/II | 0.621 | 0.542 |
| NT-proBNP <1,800 pg/mL | 0.808a | 0.521 |
| All of the above | 0.641 | 0.500 |
6MWD: 6-minute walk distance; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; ROC: receiver operating characteristics; WHO: World Health Organization.
Sufficient to use ROC as threshold.
Univariate and multivariate logistic regression analyses to determine the associations between baseline Ang-2 or sP-selectin and response to therapy at week 16 are shown in Table 5. A significant association was found between Ang-2 at baseline and NT-proBNP at week 16, with P values of 0.0002 and 0.0004 in the univariate and multivariate analyses, respectively. No other significant associations were found for Ang-2 or sP-selectin.
Table 5.
Univariate and multivariate logistic regression analysis of the relationship between angiopoietin-2 (Ang-2) or soluble P-selectin (sP-selectin) levels at baseline and response to oral treprostinil therapy at week 16
| Ang-2 at baselinea | sP-selectin at baselinea | |||
|---|---|---|---|---|
| Definition of response at week 16 | ML estimate (P value) | OR (95% CI) | ML estimate (P value) | OR (95% CI) |
| Univariate analysis: | ||||
| 6MWD change from baseline >40 m | −0.02 (0.9649) | 0.98 (0.41–2.37) | −0.55 (0.2260) | 0.58 (0.24–1.41) |
| 6MWD >380 m | −0.21 (0.6416) | 0.81 (0.34–1.94) | −0.72 (0.1119) | 0.49 (0.20–1.18) |
| NYHA/WHO functional class I/II | −0.47 (0.3032) | 0.63 (0.26–1.52) | −0.39 (0.3937) | 0.68 (0.28–1.65) |
| NT-proBNP <1,800 pg/mL | −2.07 (0.0002)c | 0.13 (0.04–0.37) | 0.04 (0.9306) | 1.04 (0.40–2.70) |
| All of the above | −0.94 (0.1133) | 0.39 (0.12–1.25) | −0.22 (0.6925) | 0.80 (0.27–2.40) |
| Multivariate analysisb: | ||||
| 6MWD change from baseline >40 m | −0.07 (0.9004) | 0.94 (0.33–2.66) | −0.13 (0.8077) | 0.88 (0.31–2.51) |
| 6MWD >380 m | 0.23 (0.7184) | 1.26 (0.35–4.53) | −0.42 (0.5097) | 0.66 (0.19–2.29) |
| NYHA/WHO functional class I/II | −0.15 (0.8015) | 0.86 (0.27–2.77) | 0.05 (0.9373) | 1.05 (0.33–3.29) |
| NT-proBNP <1,800 pg/mL | −2.54 (0.0004)c | 0.08 (0.02–0.32) | 0.04 (0.9414) | 1.05 (0.32–3.38) |
| All of the above | −1.69 (0.0866) | 0.18 (0.03–1.27) | 0.19 (0.8097) | 1.21 (0.25–5.87) |
6MWD: 6-minute walk distance; CI: confidence interval; ML: maximum likelihood; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; OR: odds ratio; WHO: World Health Organization.
Ang-2 was dichotomized (high vs. low) according to the threshold identified by receiver operating characteristics analysis (12.0 ng/mL) for response defined as NT-proBNP <1,800 pg/mL and according to the median level at baseline (9.3 ng/mL) for all other response definitions; sP-selectin was dichotomized according to the median level at baseline (64.0 ng/mL).
Controlling for baseline 6MWD, age, sex, WHO/NYHA functional class, Borg index, and disease etiology.
Significant at type I error level of 0.05.
Association of Ang-2 and sP-selectin levels with dose of oral treprostinil at week 16
Ang-2 and sP-selectin levels and their change from baseline at week 16 showed no significant difference between patients receiving low and high doses of oral treprostinil (≤3.1 mg and >3.1 mg twice daily, respectively). The median change from baseline in Ang-2 level at week 16 was −0.2 ng/mL and −0.4 ng/mL in the low- and high-dose groups, respectively (P = 0.306); the corresponding median changes in sP-selectin were 3.0 ng/mL and −3.0 ng/mL, respectively (P = 0.351). Spearman correlation analyses also found no significant association between oral treprostinil dose at week 16 and Ang-2 or sP-selectin baseline levels or change from baseline at week 16 (Table 3).
Discussion
This study explores two potential biomarkers, Ang-2 and sP-selectin, in a cohort of patients from the FREEDOM-C2 study. In total, biomarker data were available for 83 patients in the oral treprostinil group (53%) and 95 patients in the placebo group (62%). There were no significant differences in Ang-2 or sP-selectin levels between the two treatment groups at baseline or at week 16. In addition, no clinically relevant changes were seen when comparing baseline levels of Ang-2 and sP-selectin with levels at week 16; paradoxically, sP-selectin was increased from baseline to a greater extent in patients who showed a response to oral treprostinil compared with those who did not. Additional comprehensive analysis showed no significant association of Ang-2 or sP-selectin levels with functional outcome at week 16. Of note, Ang-2 was associated with the predefined treatment response of lowering the levels of a prognostic biomarker (NT-proBNP) at week 16, but Ang-2 itself was not associated with an overall clinical response to therapy.
An association of Ang-2 with NT-proBNP level has been reported in patients with acute myocardial infarction,32 but otherwise our findings contrast with those of published studies. Ang-2 levels were associated with disease severity and predicted poor outcomes in earlier studies involving patients with chronic heart failure, acute decompensated heart failure, and myocardial infarction16,17,32,33 as well as patients with PAH.7,8 In a cohort study involving patients with idiopathic PAH, Ang-2 correlated with hemodynamic parameters and mixed venous oxygen saturation, and elevated Ang-2 (>2.9 ng/mL) was an independent predictor of mortality.8 Earlier research also demonstrated a positive correlation between sP-selectin and mean pulmonary arterial pressure, total pulmonary resistance, and pulmonary vascular resistance in patients with primary pulmonary hypertension (but not in those with secondary pulmonary hypertension).23 Furthermore, Ang-2 and sP-selectin levels in patients with PAH were found to decrease following treatment with intravenous prostacyclin, and the Ang-2 decrease was associated with an improvement in 6MWD.7,23 Treatment of PAH with the endothelin receptor antagonist bosentan was also associated with a decrease in sP-selectin levels in one study,24 whereas another study showed an overall increase.25
In FREEDOM-C2, background PAH therapy was established and stable in all included patients, with the majority already receiving double combination therapy. By contrast, earlier published studies mainly included therapy-naive patients or patients for whom earlier treatment had shown minimal or no efficacy.7,8,23-25 One can speculate that the background therapy in FREEDOM-C2 had already influenced the biomarker profile at baseline, resulting in low levels that left little opportunity for further reduction by addition of oral treprostinil. Our sP-selectin data provide some support for this: the mean level in our study population at baseline was 76.6 ng/mL, compared with previously reported baseline levels of 243–367 ng/mL in patients with PAH and 99–132 ng/mL in control subjects.23,24 One other study that reported low baseline levels of sP-selectin (88.3 ng/mL in patients with PAH associated with connective tissue disease and 45.2 ng/mL in control subjects) also showed an increase in sP-selectin from baseline after study treatment.25 However, the median Ang-2 level at baseline in our study (8.7 ng/mL) was broadly consistent with baseline levels in earlier PAH studies in which Ang-2 decreased in response to treatment (6.5 ng/mL8 and ∼9 ng/mL7 [estimated from graph]). Nevertheless, one can speculate that Ang-2 and sP-selectin might be of clinical interest when oral treprostinil is given to therapy-naive patients.
Our post hoc analysis of FREEDOM-C2 data used a new, predefined composite end point that had not been previously assessed in the main study.27 The composite end point, which comprised WHO/NYHA functional class I–II, NT-proBNP <1,800 pg/mL, and relevant improvement of 6MWD at week 16, was reached by only 16 of 83 patients receiving oral treprostinil, independently of dose titration. Thus, a significant treatment response (as defined by our composite end-point criteria) was evident in only a small proportion of the patients, and the ability to detect an association of the selected biomarker with outcome is statistically limited by the small number of patients. Possible reasons for not reaching the predefined composite end point of our study include the presence of stable background PAH therapy at baseline and the relatively short duration of the trial; a longer trial may have allowed titration of the study drug to higher doses, which might have had greater efficacy, as discussed in the main publication on the FREEDOM-C2 study.27 In addition, a predefined combined end point was reached by 16.8% of patients in a large multicenter study investigating the inhalation of iloprost,34 highlighting the complexity of reaching numerous strict end points in multicenter trials.
Other limitations of the current study are the sample size and the lack of complete biomarker data from all patients, since the biomarker analysis was optional and not mandatory in the FREEDOM-C2 study.27 Considering that biomarker data were available for only 53% of the treatment group, selection bias is likely, although an analysis of key baseline characteristics found no significant differences between patients with and patients without biomarker data. Furthermore, there were no pulmonary hemodynamic data and no echocardiography data available, because these were not included in the initial study design.27 The definition of treatment response should also be considered when interpreting the results. For example, at baseline, more than one-quarter of the randomized patients in FREEDOM-C2 were already in WHO/NYHA functional class II and more than half already had NT-proBNP <1,800 pg/mL; if these patients met the response criteria for functional class and/or NT-proBNP at week 16, this may have been due to the continuation of their stable background therapy rather than a true response to the addition of oral treprostinil. Thus, the responder population may have been diluted somewhat. However, the response criterion of >40-m increase from baseline in 6MWD is more likely to reflect a true response to oral treprostinil, and this also showed no relationship with Ang-2 levels.
In conclusion, the results of our analysis of patients with PAH who had available biomarker data in the FREEDOM-C2 study suggest that Ang-2 and sP-selectin are not associated with treatment response when adding oral treprostinil in addition to stable monotherapy or dual combination therapy. This finding per se has a certain clinical relevance, because it mirrors routine clinical practice for patients already receiving PAH therapy.
Acknowledgments
We thank Claire Mulligan (Beacon Medical Communications, Brighton, UK) for editorial support, funded by United Therapeutics. Data were provided by the United Therapeutics Study Data Query Program.
Source of Support: This work was supported by United Therapeutics, which provided editorial assistance.
Conflict of Interest: MJR has received support from United Therapeutics and Bayer Pharma and speaker fees from Actelion, Mundipharma, Roche, and United Therapeutics. RS has received honoraria from Actelion, Pfizer, Bayer-Schering, Novartis, and Solvay and research grant support from Bayer Schering, Actelion, Pfizer, Solvay, and Ergonex. WS has received speaker/consultancy fees from Pfizer and Bayer Pharma. YR is an employee of United Therapeutics. HAG has received consultancy fees from Bayer, Actelion, Pfizer, Merck, GlaxoSmithKline (GSK), and Novartis; fees for participation in advisory boards from Bayer, Pfizer, GSK, Actelion, and Takeda; lecture fees from Bayer HealthCare, GSK, Actelion, and Encysive/Pfizer; industry-sponsored grants from Bayer HealthCare, Aires, Encysive/Pfizer, and Novartis; and sponsored grants from the German Research Foundation, Excellence Cluster Cardiopulmonary Research, and the German Ministry for Education and Research. HG has received fees from Actelion, AstraZeneca, Bayer, GSK, Janssen-Cilag, Lilly, Novartis, OMT, Pfizer, and United Therapeutics.
References
- 1.Rosenthal JL, Jacob MS. Biomarkers in pulmonary arterial hypertension. Curr Heart Fail Rep 2014;11(4):477–484. [DOI] [PubMed]
- 2.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016;37(1):67–119. [DOI] [PubMed]
- 3.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. J Am Coll Cardiol 2009;53(17):1573–1619. [DOI] [PubMed]
- 4.Rosenberg M, Meyer FJ, Gruenig E, Lutz M, Lossnitzer D, Wipplinger R, Katus HA, Frey N. Osteopontin predicts adverse right ventricular remodelling and dysfunction in pulmonary hypertension. Eur J Clin Invest 2012;42(9):933–942. [DOI] [PubMed]
- 5.Santos M, Reis A, Goncalves F, Ferreira-Pinto MJ, Cabral S, Torres S, Leite-Moreira AF, Henriques-Coelho T. Adiponectin levels are elevated in patients with pulmonary arterial hypertension. Clin Cardiol 2014;37(1):21–25. [DOI] [PMC free article] [PubMed]
- 6.Foris V, Kovacs G, Tscherner M, Olschewski A, Olschewski H. Biomarkers in pulmonary hypertension: what do we know? Chest 2013;144(1):274–283. [DOI] [PubMed]
- 7.Hiremath J, Thanikachalam S, Parikh K, Shanmugasundaram S, Bangera S, Shapiro L, Pott GB, et al. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trial. J Heart Lung Transplant 2010;29(2):137–149. [DOI] [PubMed]
- 8.Kumpers P, Nickel N, Lukasz A, Golpon H, Westerkamp V, Olsson KM, Jonigk D, et al. Circulating angiopoietins in idiopathic pulmonary arterial hypertension. Eur Heart J 2010;31(18):2291–2300. [DOI] [PubMed]
- 9.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 2004;103(11):4150–4156. [DOI] [PubMed]
- 10.Mandriota SJ, Pyke C, Di Sanza C, Quinodoz P, Pittet B, Pepper MS. Hypoxia-inducible angiopoietin-2 expression is mimicked by iodonium compounds and occurs in the rat brain and skin in response to systemic hypoxia and tissue ischemia. Am J Pathol 2000;156(6):2077–2089. [DOI] [PMC free article] [PubMed]
- 11.Pichiule P, Chavez JC, LaManna JC. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J Biol Chem 2004;279(13):12171–12180. [DOI] [PubMed]
- 12.Fiedler U, Krissl T, Koidl S, Weiss C, Koblizek T, Deutsch U, Martiny-Baron G, Marme D, Augustin HG. Angiopoietin-1 and angiopoietin-2 share the same binding domains in the Tie-2 receptor involving the first Ig-like loop and the epidermal growth factor-like repeats. J Biol Chem 2003;278(3):1721–1727. [DOI] [PubMed]
- 13.Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci 2005;118(Pt 4):771–780. [DOI] [PubMed]
- 14.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A 2002;99(17):11205–11210. [DOI] [PMC free article] [PubMed]
- 15.Chong AY, Caine GJ, Freestone B, Blann AD, Lip GY. Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor tie-2 levels in congestive heart failure. J Am Coll Cardiol 2004;43(3):423–428. [DOI] [PubMed]
- 16.Eleuteri E, Di Stefano A, Tarro Genta F, Vicari C, Gnemmi I, Colombo M, Mezzani A, Giannuzzi P. Stepwise increase of angiopoietin-2 serum levels is related to haemodynamic and functional impairment in stable chronic heart failure. Eur J Cardiovasc Prev Rehabil 2011;18(4):607–614. [DOI] [PubMed]
- 17.Poss J, Ukena C, Kindermann I, Ehrlich P, Fuernau G, Ewen S, Mahfoud F, Kriechbaum S, Bohm M, Link A. Angiopoietin-2 and outcome in patients with acute decompensated heart failure. Clin Res Cardiol 2015;104(5):380–387. [DOI] [PubMed]
- 18.Lemieux C, Maliba R, Favier J, Theoret JF, Merhi Y, Sirois MG. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood 2005;105(4):1523–1530. [DOI] [PubMed]
- 19.Maliba R, Brkovic A, Neagoe PE, Villeneuve LR, Sirois MG. Angiopoietin-mediated endothelial P-selectin translocation: cell signaling mechanisms. J Leukoc Biol 2008;83(2):352–360. [DOI] [PubMed]
- 20.Valentijn KM, Sadler JE, Valentijn JA, Voorberg J, Eikenboom J. Functional architecture of Weibel-Palade bodies. Blood 2011;117(19):5033–5043. [DOI] [PMC free article] [PubMed]
- 21.Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J 1995;9(10):866–873. [PubMed]
- 22.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J 2003;24(24):2166–2179. [DOI] [PubMed]
- 23.Sakamaki F, Kyotani S, Nagaya N, Sato N, Oya H, Satoh T, Nakanishi N. Increased plasma P-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation 2000;102(22):2720–2725. [DOI] [PubMed]
- 24.Iannone F, Riccardi MT, Guiducci S, Bizzoca R, Cinelli M, Matucci-Cerinic M, Lapadula G. Bosentan regulates the expression of adhesion molecules on circulating T cells and serum soluble adhesion molecules in systemic sclerosis-associated pulmonary arterial hypertension. Ann Rheum Dis 2008;67(8):1121–1126. [DOI] [PMC free article] [PubMed]
- 25.Cella G, Vianello F, Cozzi F, Marotta H, Tona F, Saggiorato G, Iqbal O, Fareed J. Effect of bosentan on plasma markers of endothelial cell activity in patients with secondary pulmonary hypertension related to connective tissue diseases. J Rheumatol 2009;36(4):760–767. [DOI] [PubMed]
- 26.Semenov AV, Kogan-Ponomarev M, Ruda M, Komarov AL, Panchenko EP, Chazova IE, Mazurov AV. Soluble P-selectin: a marker of platelet activation and vessel wall injury: increase of soluble P-selectin in plasma of patients with myocardial infarction, massive atherosclerosis and primary pulmonary hypertension [in Russian]. Ter Arkh 2000;72(4):15–20. [PubMed]
- 27.Tapson VF, Jing ZC, Xu KF, Pan L, Feldman J, Kiely DG, Kotlyar E, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest 2013;144(3):952–958. [DOI] [PubMed]
- 28.Mathai SC, Puhan MA, Lam D, Wise RA. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;186(5):428–433. [DOI] [PMC free article] [PubMed]
- 29.Gabler NB, French B, Strom BL, Palevsky HI, Taichman DB, Kawut SM, Halpern SD. Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation 2012;126(3):349–356. [DOI] [PMC free article] [PubMed]
- 30.Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J 2005;26(5):858–863. [DOI] [PubMed]
- 31.McLaughlin VV, Gaine SP, Howard LS, Leuchte HH, Mathier MA, Mehta S, Palazzini M, Park MH, Tapson VF, Sitbon O. Treatment goals of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl):D73–D81. [DOI] [PubMed]
- 32.Chen S, Guo L, Chen B, Sun L, Cui M. Association of serum angiopoietin-1, angiopoietin-2 and angiopoietin-2 to angiopoietin-1 ratio with heart failure in patients with acute myocardial infarction. Exp Ther Med 2013;5(3):937–941. [DOI] [PMC free article] [PubMed]
- 33.Lukasz A, Beutel G, Kumpers P, Denecke A, Westhoff-Bleck M, Schieffer B, Bauersachs J, Kielstein JT, Tutarel O. Angiopoietin-2 in adults with congenital heart disease and heart failure. PLoS ONE 2013;8(6):e66861. [DOI] [PMC free article] [PubMed]
- 34.Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002;347(5):322–329. [DOI] [PubMed]