Figure 3. NLK mediates osmotic stress signal to induce YAP nuclear localization.
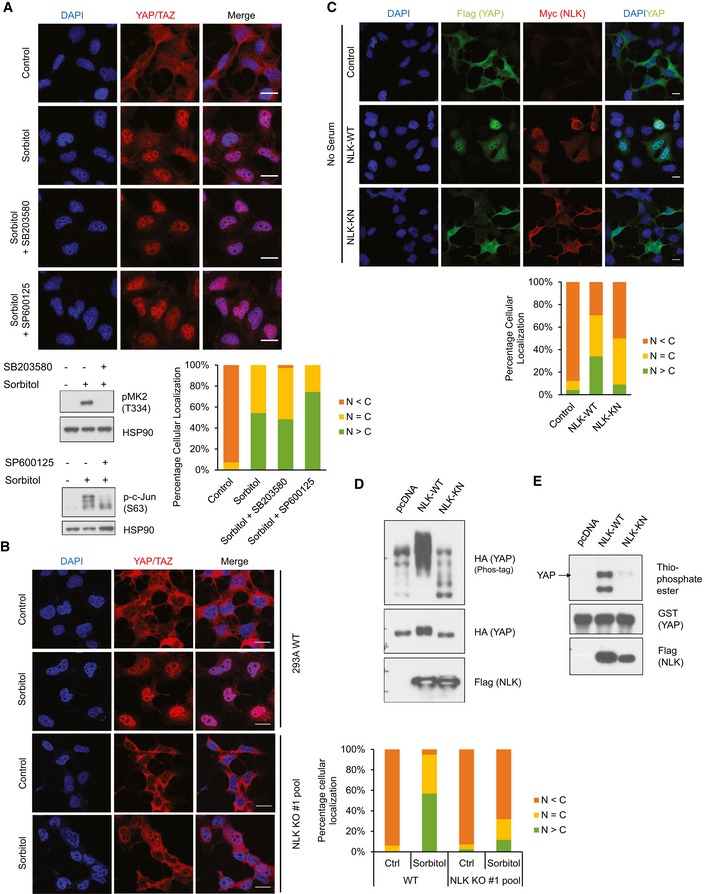
- Inhibition of p38 or JNK does not block osmotic stress‐induced YAP nuclear localization. HEK293A cells were pretreated with 2 μM p38 inhibitor (SB203580) or 20 μM JNK inhibitor (SP600125) before treatment followed by 0.2 M sorbitol for 1 h in the absence of serum. Quantification of YAP/TAZ subcellular staining is shown. Scale bars: 20 μm. Cell lysates from identically treated samples were examined for phosphorylation of p38 substrate MK‐2 and JNK substrate c‐Jun.
- NLK knockout blocks osmotic stress‐induced YAP nuclear localization. HEK293A cells were transiently transfected with CRISPR/Cas9 to knock out NLK. Wild‐type (WT) cells and the NLK KO cell pool were treated with 0.2 M sorbitol for 1 h in the absence of serum. YAP/TAZ subcellular localization was determined by immunofluorescence staining. Scale bars: 20 μm.
- NLK induces YAP nuclear translocation. HEK293A cells were co‐transfected with Flag‐YAP together with vector control, Myc‐NLK‐WT (wild‐type NLK), or NLK‐KN (kinase‐negative mutant). Cells were serum starved for 6 h, and YAP localization and NLK expression were determined by Flag (green) and Myc (red) antibodies, respectively. Scale bars: 20 μm.
- NLK induces YAP phosphorylation. HA‐YAP was co‐transfected with NLK‐WT or NLK‐KN in HEK293A cells. The phos‐tag gel showed NLK‐WT but not NLK‐KN caused a significant mobility shift of YAP.
- NLK phosphorylates YAP in vitro. NLK‐WT and NLK‐KN were immunoprecipitated from HEK293A cells, and an in vitro kinase assay was performed using recombinant GST‐YAP as a substrate in the presence of ATP‐γ‐S. Total phosphorylation of YAP was detected by immunoblotting with thiophosphate ester antibody, which identifies the alkylated thiophosphorylation on YAP.
Source data are available online for this figure.
