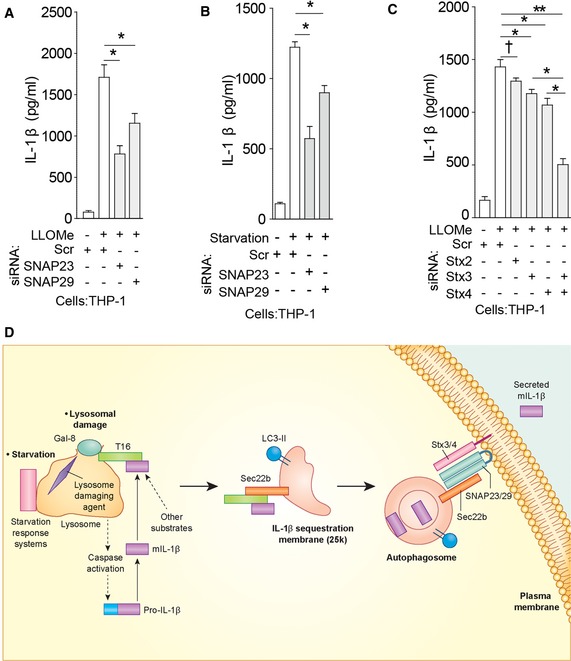
Secretory autophagy pathway. Upon lysosomal damage caused by inflammasome agonists such as silica, alum, monosodium urate, and Leu‐Leu‐O‐Me, inflammasome activation leads to caspase‐1‐dependent processing of cytosolic pro‐IL‐1β into mature IL‐1β (mIL‐1β). The autophagy regulatory systems (starvation/nutrition response systems: mTOR, LYNUS, TFEB) located at the lysosome transduce starvation signals (Napolitano & Ballabio,
2016). Upon lysosomal damage and stress, galectin‐8 recognizes β‐galactosides on the exposed lumenal membrane and/or glycosylated lysosomal lumenal proteins released into the cytosol and activates the galectin‐8–TRIM16 complex. TRIM16 binds secretory autophagy cargo (mIL‐1β and potentially other cargo). TRIM16 forms complexes with Sec22b to transfer the cargo to the autophagy‐induced LC3‐II
+ membrane 25 k carriers (a direct interaction between Sec22b and TRIM16 remains to be determined). Depiction of mIL‐1β between the two membranes or within the lumen of the double membrane‐delimited autophagosomal intermediates reflects the sequestration processes that may be involved: IL‐1β is sequestered either through the reported Hsp90‐dependent import across the delimiting membrane into the lumen of carriers topologically corresponding to the intermembrane space in conventional autophagosomes (Zhang
et al,
2015), or is sequestered into the inner lumen of conventional double‐membrane autophagosomal organelles, specializing in secretion, simultaneously observed in the same study (Zhang
et al,
2015). Not depicted is the compatibility of TRIM16's receptor function with both of the above processes, that is, either a hand‐over of the cargo to Hsp90 or cargo sequestration via TRIM16 acting as a receptor/adaptor directly interacting with mAtg8s as previously reported (Mandell
et al,
2014); the latter option is in keeping with the observations that TRIM16 is co‐secreted with IL‐1β (Munding
et al,
2006). At this point, Sec22b molecules (acting as R‐SNAREs) on the delimiting membrane facing the cytosol carry out fusion at the plasma membrane in conjunction with the Qbc‐SNAREs SNAP‐23 and SNAP‐29 and plasma membrane Qa‐SNAREs syntaxin 3 and syntaxin 4, thus delivering IL‐1β to the extracellular milieu where it exerts its biological function.