Abstract
Background
Supraventricular tachycardia (SVT) is common in complex congenital heart disease (CCHD) patients with single‐ventricle physiology and may cause hemodynamic deterioration. We reported the outcomes of catheter ablation for such complex SVT in these single‐ventricle CCHD patients.
Methods and Results
Patients with single‐ventricle physiology (defined as CCHD patients) who received electrophysiology studies and catheter ablation between 1995 and 2015 were studied. We enrolled 30 CCHD patients (18 with right atrial isomerism, 5 with left atrial isomerism, and 7 with other CCHDs; 17 male, 13 female). The age of onset of clinical SVT was 6.7 years (±4.7 years). Electrophysiology studies and ablation were performed at age 7.1 years (±3.9 years); body weight was 20.7 kg (±10.0 kg). Twin atrioventricular nodes were present in 60% of patients (right atrial isomerism, 72.2%; left atrial isomerism, 40%; other CCHDs, 42.9%). Manifested preexcitation was noted in 10% of patients. SVT was induced in 21 patients. Twin atrioventricular nodal reentrant tachycardia was the most common (57.1%), followed by atrioventricular reentrant tachycardia (28.6%), junctional tachycardia (14.3%), and atrioventricular nodal reentrant tachycardia (9.5%). Multiple arrhythmias were common (33.3%), particularly in patients with atrioventricular reentrant tachycardia (50%). Ablation successfully eliminated SVT in 12 of 14 patients (85.7%), with a recurrence rate of 16.7% during 6 years of follow‐up.
Conclusions
Transcatheter ablation of complex SVT substrates, including minor atrioventricular node of twin atrioventricular nodal reentrant tachycardia, accessory pathways of atrioventricular reentrant tachycardia, and a slow pathway of atrioventricular nodal reentrant tachycardia, is effective in CCHD patients. The limitations are limited vascular access and the risk of atrioventricular block.
Keywords: catheter ablation, congenital heart disease, heterotaxy, single ventricle, supraventricular tachycardia
Subject Categories: Congenital Heart Disease, Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator
Introduction
Patients with complex congenital heart disease (CCHD) with single‐ventricle physiology need to be finally palliated by Fontan‐type operation.1, 2 In Fontan circulation, systemic venous blood flow is routed directly to the pulmonary circulation, totally bypassing the right ventricle, and the flow from the systemic veins through the bilateral lungs and back to the ventricle is entirely passive. Because of this unique physiology, patients with Fontan circulation are particularly intolerant of tachyarrhythmia with a short diastolic interval.3 Atrial arrhythmia is the most common arrhythmia in such patients, and several studies have demonstrated that atrial tachyarrhythmia is associated with increased morbidity and mortality.3, 4, 5 In addition to atrial arrhythmia, supraventricular tachycardia (SVT) with substrates that are unique in CCHD patients can cause hemodynamic deterioration and Fontan failure.3, 6
Catheter ablation is effective for eliminating SVT (ie, atrioventricular reentrant tachycardia [AVRT] or atrioventricular nodal reentrant tachycardia [AVNRT]) in children without structural heart disease.7 In CCHD patients, however, the location and characteristics of the atrioventricular node are often complex, and twin atrioventricular nodes (TAVN) may occur in some patients.8, 9 Consequently, catheter ablation for SVT in CCHD patients is more challenging, particularly in small children. In this paper, we presented our experience to elucidate the electrophysiological characteristics and outcomes of catheter ablation in CCHD patients.
Methods
Patients with single‐ventricle physiology (defined as CCHD patients) who underwent an electrophysiology study (EPS) for evaluation of SVT at National Taiwan University Hospital during the period from 1995 to 2015 were enrolled. Medical charts were reviewed, and electrophysiology data were retrieved. The study was approved by the institutional review board of our hospital, and the requirement of informed consent was waived. SVT was defined as reentry arrhythmia involving atrioventricular nodal tissue (including atrioventricular node and bundle of His conduction system) or accessory pathways.
Electrophysiology Studies
We performed the EPSs under intravenous anesthesia with propofol, and antiarrhythmic agents were discontinued for 2 days, except for beta blockers. Biplane fluoroscopy was used for all EPSs. We first performed ventriculography to define the precise location of the atrioventricular ring. We then introduced 2 or 3 mapping catheters from the femoral veins or internal jugular vein, 1 quadripolar fixed catheter at the right atrium, and 1 multipolar fixed catheter (steerable decapolar mapping catheter or EPstar His‐RV Fixed catheter [J‐Cath]) at the right ventricle with or without 1 quadripolar fixed catheter to define the bundle of His area. For patients with limited vascular access, we used 1 quadripolar fixed catheter at the right atrium and 1 multipolar catheter to record the right ventricle and His‐bundle signals.
We mapped the bundle of His potential around the whole atrioventricular ring by using the fixed catheter or steerable ablation catheter. A basic EPS was then performed using standard atrial and ventricular rapid pacing and extrastimulation. Atrial pacing was performed from both the right and left atria. The 12‐lead ECG was verified at different pacing cycle lengths to detect a shift in QRS morphology. Arrhythmia inducibility was determined in all patients. When arrhythmia could not be induced with the standard protocol, isoproterenol infusion was given to enhance inducibility (if tolerated). For those with induced tachycardia, we introduced (or exchanged when vascular access was limited) an ablation catheter for mapping. Antegrade and retrograde atrioventricular conduction were delineated carefully through meticulous mapping.
TAVN was defined if (1) at least 2 nonpreexcited QRS morphologies were recorded at 2 different sites, (2) His‐bundle potential was recorded at 2 discrete sites, and (3) decremental atrioventricular conduction was defined at these 2 bundle of His areas.10 The major atrioventricular node was defined as the antegrade atrioventricular conduction at sinus rhythm, and the other atrioventricular nodes were defined as minor atrioventricular nodes. For the patients with an alternative or fusion ECG pattern during sinus rhythm, the major atrioventricular node was defined as the antegrade atrioventricular conduction during dominant SVT. Twin atrioventricular nodal reentrant tachycardia (TAVNRT) was defined if 2 atrioventricular nodes were recorded after reentrant tachycardia induction with antegrade conduction through 1 atrioventricular node and retrograde conduction through the other atrioventricular node. Junctional tachycardia was defined as narrow QRS tachycardia with the same morphology as atrial pacing but with dissociated atrioventricular conduction.11
Ablation
After localization, radiofrequency energy was delivered through ablation catheters. For accessory pathway–related AVRT, we targeted the accessory pathways (VA fusion or earliest retrograde A signal). For TAVNRT, we targeted the retrograde‐conducted minor atrioventricular node (the earliest retrograde A signal). We used the temperature control mode with the highest temperature limit ranging from 60°C to 65°C and the highest energy given at 50 W. The therapeutic end point was noninducibility of tachycardia and elimination of the accessory pathway or minor atrioventricular node. Patients received heparinization during the procedure and overnight after the EPS, followed by aspirin at 3 to 5 mg/kg per day for 2 weeks.
At follow‐up, any cardiac symptoms, including palpitation, chest discomfort, and near‐syncope episodes, were recorded from the medical chart. The ECG was also examined carefully to confirm the existence of 2 QRS morphologies.
Statistical Analyses
SPSS version 15.0 (IBM Corp) was used to perform statistical analysis. All continuous variables were expressed as mean±SD, and categorical variables were expressed as number (percentage). Data from the independent group were compared using the nonparametric Kruskal–Wallis test for continuous data and the chi‐square test for categorical data. P<0.05 was considered statistically significant.
Results
Basic Clinical Characteristics
We enrolled 30 CCHD patients with single‐ventricle physiology (17 male, 13 female). We categorized these patients into 3 groups according to the underlying cardiac anomaly: right atrial isomerism (RAI), left atrial isomerism (LAI), and other CCHDs. Table 1 presents the underlying cardiac anomaly in these 3 groups. All patients with RAI and most with LAI exhibited similar combinations of cardiac anomalies including a common atrioventricular canal and double‐outlet right ventricle with or without left ventricular hypoplasia. Patients with other CCHDs exhibited varied cardiac anomalies. Table 2 lists the basic clinical characteristics. The mean age of onset of clinical SVT was 6.7 years. The mean age at Fontan or total cavopulmonary circulation surgery was 8.4 years. The age at surgery has decreased gradually over the past 20 years because of better outcomes for early total cavopulmonary circulation surgery in recent reports.12 Because transcatheter ablation is difficult after Fontan‐type surgery (the only route from the vena cava to the atrium after Fontan surgery is fenestration of the conduit), we preferred performing an EPS and transcatheter ablation before Fontan surgery. Only 2 of these patients received an EPS after Fontan surgery. The mean age at the time of the EPS was 7.1 years, and the mean body weight was 20.7 kg in our study population.
Table 1.
Cardiac Anomalies of the 3 Groups
| Group (n) | Cardiac Anomaly, n |
|---|---|
| RAI (18) |
Common atrioventricular canal, 18 Double outlet right ventricle, 18 Total anomalous pulmonary venous return, 18 Supracardiac type, 4; infracardiac type, 1; cardiac type, 13 Pulmonary stenosis or atresia, 18 Pulmonary stenosis, 15; pulmonary atresia, 3 Unbalanced ventricle, 8 Dextrocardia, 6; mesocardia, 1 |
| LAI (5) |
Common atrioventricular canal, 4 Double outlet right ventricle, 4 Pulmonary stenosis, 4 Unbalanced ventricle, 2 Dextrocardia, 2 |
| Other CCHD (7) |
Double‐inlet left ventricle, Holmes heart, 1 Pulmonary atresia with intact ventricular septum, 1 Situs inversus, double‐outlet right ventricle, ASD, VSD with unusual septal alignment, pulmonary atresia, 1 Crisscross heart, ccTGA, large ventricle septal defect, 1 ccTGA, large ventricle septal defect, 2 TGA, large ventricle septal defect, pulmonary atresia, 1 |
ASD indicates atrial septal defect; CCHD, complex congenital heart disease; ccTGA, congenitally corrected transposition of the great arteries; LAI, left atrial isomerism; RAI, right atrial isomerism; TGA, transposition of the great arteries; VSD, ventricular septal defect.
Table 2.
Basic Clinical Characteristics of the 3 Patient Groups
| All | RAI | LAI | Other CCHD | P Value | |
|---|---|---|---|---|---|
| Patients, n | 30 | 18 | 5 | 7 | |
| Sex, male/female, n/n | 17/13 | 11/7 | 1/4 | 5/2 | 0.173 |
| Age of onset of clinical SVT, ya | 6.7±4.7 | 4.8±3.0 | 6.1±3.6 | 11.7±5.4 | 0.016 |
| Age at EPS, y | 7.1±3.9 | 5.8±1.9 | 6.3±4.1 | 10.8±5.6 | 0.070 |
| Surgical state at EPS | 0.593 | ||||
| Before Fontan operation | 28 (93) | 17 (94) | 5 (100) | 6 (86) | |
| After Fontan operation | 2 (7) | 1 (6) | 0 | 1 (14) | |
| Final completion of Fontan/TCPC operation | 24 (80) | 13 (72.2) | 4 (80) | 7 (100) | 0.297 |
| Age at Fontan/TCPC operation, y | 8.4±4.0 | 7.1±2.1 | 8.4±4.3 | 11.0±5.6 | 0.221 |
| Body weight at EPS, kg | 20.7±10.0 | 17.7±5.1 | 19.8±9.0 | 29.1±15.6 | 0.152 |
| ECG pattern | |||||
| >2 narrow QRS | 10 (33.3) | 8 (44.4) | 0 | 2 (28.6) | 0.168 |
| Manifest preexcitation | 3 (10) | 2 (11.1) | 1 (20) | 0 | 0.507 |
| Indication of EPS | 0.128 | ||||
| Clinical confirmed SVT | 13 (43.3) | 7 (38.9) | 1 (20) | 5 (71.4) | |
| Clinical SVT without 12‐lead ECG confirmation | 10 (33.3) | 8 (44.4) | 1 (20) | 1 (14.3) | |
| Preoperative assessment | 7 (23.3) | 3 (16.7) | 3 (60) | 1 (14.3) | |
Data are shown as mean±SD or number (percentage) except as noted. CCHD indicates complex congenital heart disease; EPS, electrophysiology study; LAI, left atrial isomerism; RAI, right atrial isomerism; SVT, supraventricular tachycardia; TCPC, total cavopulmonary circulation.
Only patients with clinical SVT were included.
Electrophysiology Studies
Table 3 describes the electrophysiological characteristics. The indications for an EPS included clinically confirmed SVT (with 12‐lead ECG confirmation) in 13 patients (43.3%; most presented with palpitation and dizziness or near syncope), clinical SVT without 12‐lead ECG in 10 patients (33.3%; mostly shown on the ECG monitor during hospitalization for surgery, cardiac catheterization, or other reasons), and preoperative assessment in 7 patients (23.3%; including suspected TAVN or abnormal atrioventricular node function at previous ECG examination). Overall, >2 narrow QRS ECG patterns were common (n=10, 33.3%) in the CCHD patients, particularly those with RAI and with other CCHDs. Among these 10 patients, 4 (40%) had fused QRS morphology (Figure 1A), and the other 6 (60%) had 1 dominant ECG pattern, but another ECG pattern may occur occasionally. During the EPSs, more than half of the patients (n=18, 60%) had TAVN (inclusive of 8 patients with 2 ECG patterns demonstrated only at EPS), particularly the RAI patients (72.2%) (Figure 1B). The major atrioventricular node occurred more commonly at the posterior atrioventricular ring but also presented at the anterior atrioventricular ring (Figure 2). In the 7 patients without heterotaxy syndrome, 3 (42.9%) had TAVN. Of those, 2 had congenitally corrected transposition of the great arteries, and 1 had a double‐outlet right ventricle with situs inversus, dextrocardia, atrial septal defect, large ventricular septal defect with unusual atrioventricular septal alignment, and pulmonary atresia.
Table 3.
Electrophysiological Characteristics in the 3 Patient Groups
| All | RAI | LAI | Other CCHDs | P Value | |
|---|---|---|---|---|---|
| EPS, n | 30 | 18 | 5 | 7 | |
| Twin AV node | 18 (60) | 13 (72.2) | 2 (40) | 3 (42.9) | 0.245 |
| Wenckebach cycle length, ms | 276±63 | 252±26 | 378±110 | 278±31 | 0.011 |
| SVT | 21 (70.0) | 14 (77.8) | 2 (40) | 5 (71.4) | 0.263 |
| TAVNRT | 12 (57.1) | 9 (64.3) | 1 (50) | 2 (40) | |
| Junctional tachycardia | 3 (14.3) | 2 (14.3) | 1 (50) | 0 | |
| AVRT | 6 (28.6) | 3 (21.4) | 1 (50) | 2 (40) | |
| AVNRT | 2 (9.5) | 1 (7.1) | 0 | 1 (20) | |
| Multiple mechanisms | 7 (33.3) | 5 (35.7) | 1 (50) | 1 (20) | 0.760 |
| Tachycardia cycle length, ms | 330±82 | 305±72 | 387±67 | 364±93 | 0.569 |
| Ablation (number) | 14 | 9 | 1 | 4 | |
| Ablation target | 0.168 | ||||
| Minor AV node | 10 (71.4) | 8 (88.8) | 1 (100) | 1 (25) | |
| Accessory pathway | 3 (21.4) | 1 (11.1) | 0 | 2 (50) | |
| Slow pathway (dual AV node) | 1 (7.1) | 0 | 0 | 1 (25) | |
| Success rate of ablation, % (n/n) | 85.7 (12/14) | 88.9 (8/9) | 0 (0/1) | 100 (4/4) | 0.034 |
| SVT recurrence, % (n/n) | 16.7 (2/12) | 25 (2/8) | — | 0 (0/4) | 0.515 |
Data are shown as mean±SD or number (percentage) except as noted. AV indicates atrioventricular; AVNRT, atrioventricular nodal reentrant tachycardia; AVRT, atrioventricular reentrant tachycardia; CCHD, complex congenital heart disease; EPS, electrophysiology study; LAI, left atrial isomerism; RAI, right atrial isomerism; SVT, supraventricular tachycardia; TAVNRT, twin atrioventricular nodal reentrant tachycardia.
Figure 1.

Electrocardiogram of twin atrioventricular node. A, A patient with right atrial isomerism who had fused two non‐preexcited QRS morphologies with different axis at usual surface ECG recording. She was documented to have twin atrioventricular nodes (TAVN) at later electrophysiology study (EPS). B, another patient with right atrial isomerism who had another QRS morphology (arrow) at the returning beat of atrial extrastimulation during EPS, suggesting the existence of TAVN.
Figure 2.

Illustration of the distribution of atrioventricular (AV) conduction tissue in our single ventricle disease patients. The major AV nodes are mostly located in the posterior site and minor AV nodes at the anterior site. However, some variations are observable. Black circles indicate major AV nodes, empty circles indicate minor AV nodes, crosses indicate accessory pathways, and triangles indicate intra‐Hisian sling connection or ectopic junctional focus.
The Wenckebach cycle length was prolonged in the LAI group compared with the other 2 groups. One LAI patient was implanted with a permanent pacemaker because of extreme bradycardia at age 7 years.
SVT could be induced in 21 of 30 patients (70%), and 2 patients (1 with RAI and 1 with other CCHD) had concomitant atrial tachyarrhythmia. TAVNRT accounted for 64.3% of SVT in the RAI patients (Figure 3) and 40% of SVT in those with other CCHDs (Figure 4). AVRT was the second most common SVT in the CCHD patients and often was associated with multiple accessory pathways (3 of 6, 50%). Manifest preexcitation was noted in 3 patients (10%). Junctional tachycardia occurred in 3 patients (14.3%), 2 with TAVN and another with a single atrioventricular node. AVNRT occurred in 2 patients (9.5%); one had typical AVNRT (and RAI), and the other had atypical AVNRT (in transposition of the great artery with a large ventricular septal defect and pulmonary atresia). Multiple reentry circuits were common (n=7, 33.3%) in these CCHD patients.
Figure 3.

Electrophysiology and ablation for twin atrioventricular nodal reentrant tachycardia in a patient with right atrial isomerism. She received electrophysiology studies at the age of 4 years. (A) and (C) In the left panel, we can see antegrade His signal at the ablation catheter (ABLd) and located in the right posterior area (arrow), which marked the location of antegrade major AV node. (B) and (D) In the right panel, during tachycardia, we can see retrograde His signal at the ablation catheter (ABLd), which was also at the nearby location of earliest retrograde A (not shown in this tracing). The ablation catheter was at the right anterior AV groove, which was the common site of minor AV nodes. Radiofrequency energy applied at this site successfully eliminated this twin AV nodal reentrant tachycardia. The black sketch indicated the atrioventricular valve area. AV indicates atrioventricular.
Figure 4.
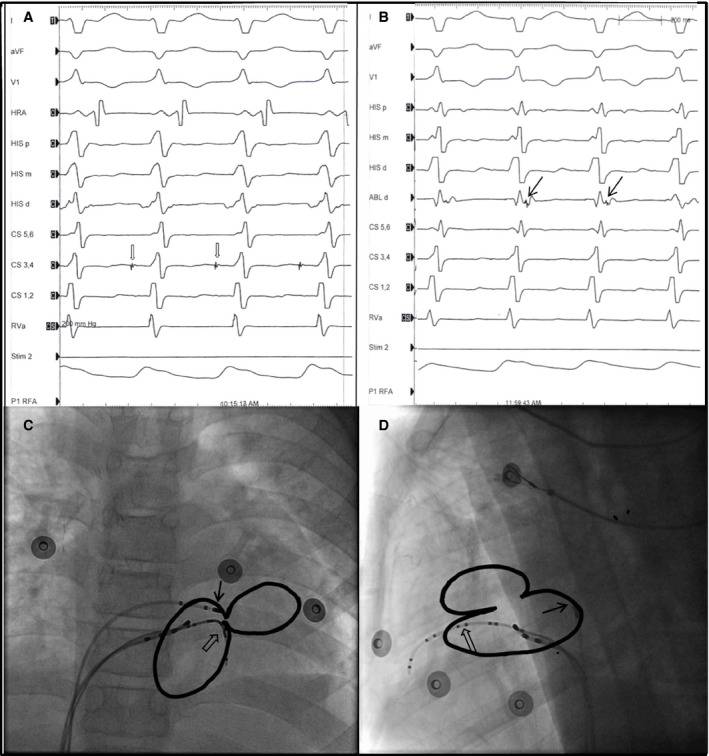
Electrophysiology and ablation for twin atrioventricular nodal reentrant tachycardia in a patient with single ventricle disease. (A) and (B) This 9‐year old boy is a patient of criss‐cross heart, corrected transposition of the great artery, large ventricular septal defect, and pulmonary atresia. During SVT, we observed an antegrade His signal at CS 3,4 catheter (A, blank arrow) and earliest retrograde A with a retrograde His signal at the ABLd catheter (B, arrow). (C) Anterior‐posterior projection. (D) Lateral projection of the catheter location. The antegrade atrioventricular (AV) node is at the anterior AV groove (arrow), and the retrograde AV node is at the posterior AV groove (blank arrow). Radiofrequency ablation at the posterior site successfully eliminated the retrograde AV node and terminated the twin AV nodal reentrant tachycardia.
Transcatheter Ablation
Transcatheter ablation through the limited catheter approach13 was performed in 14 of 21 SVT patients (66.7%). The reasons for no ablation included limited vascular access in 3 patients, parental concern about the risk of atrioventricular block in 3 patients, and hemodynamic deterioration at the time of EPS in 1 patient. Transcatheter ablation successfully eliminated SVT in 85.7% of patients. Transcatheter ablation failed in 2 patients, both of whom had multiple arrhythmias. One patient had LAI with TAVNRT, Mahaim fibers, and antidromic AVRT. Radiofrequency catheter ablation eliminated 1 SVT but failed to ablate antidromic AVRT. The other patient had RAI with TAVN, TAVNRT, and junctional tachycardia. Because of young age and a limited catheter approach, 1 pattern of SVT could be eliminated, but the other persisted; therefore, the success rate of catheter ablation was 83.3% for both TAVNRT and AVRT. For catheter ablation of TAVNRT, during radiofrequency energy application, junctional ectopic tachycardia could be induced in 6 of 8 patients. The QRS morphology at surface ECG did not change after successful ablation of minor atrioventricular nodes in the patients with 1 dominant ECG pattern. For the 4 patients with fused QRS morphology at surface ECG, successful ablation was achieved in 2, and QRS duration was not prolonged after ablation.
After a mean follow‐up duration of 6 years (median 5.1 years, range 0.2–15.7 years) after successful catheter ablation, 2 patients (16.7%) exhibited SVT recurrence. One patient who received transcatheter ablation through fenestration had the same arrhythmia recurrence 2.2 years later. The other patient, who had 3 accessory pathways ablated, exhibited SVT recurrence 1 year after ablation. Repeated EPSs showed another accessory pathway that was not detected at the initial EPS and was successfully eliminated at the second ablation.
Discussion
Reports concerning EPSs and the ablation of complex SVT in CCHD patients with single‐ventricle physiology are limited. In the present study, TAVNRT was common not only in the RAI patients but also in those with other CCHDs. Other forms of SVT, namely, AVRT from accessory pathways and AVNRT, accounted for <50% of SVT cases. Transcatheter ablation targeting the minor atrioventricular node for TAVNRT, multiple accessory pathways for AVRT, and a slow pathway for AVNRT was effective and should be performed before Fontan completion.
CCHD Patients Have a Higher Risk of SVT and May Exhibit Worse Outcomes
Fontan‐type surgery has markedly improved the long‐term outcomes of patients with single‐ventricle physiology. After Fontan surgery, the current 10‐year survival rate can reach 75% to 89%14; however, a certain percentage of patients still exhibit early Fontan failure and even mortality. Several risk factors have been proposed, and clinical arrhythmia has been shown to be one of the most crucial.3 Common arrhythmias after Fontan surgery include atrial arrhythmia, SVT, and ventricular arrhythmia. Many reports have discussed the impact of atrial arrhythmia on Fontan circulation, and successful catheter ablation can improve the clinical outcome, although recurrence is a concern.4, 5 Nevertheless, studies concerning the presence of SVT in CCHD patients receiving Fontan‐type surgery are scant. In CCHD patients, because of the varied atrioventricular conduction system, various types of SVT unrelated to the atrial scar from Fontan surgery can occur.
Heterotaxy syndrome is the most common CCHD associated with SVT. In a meta‐analysis of heterotaxy syndrome, the incidence of arrhythmia was shown to be >70% by age 5 years.15 In another large cohort study (n=257), Wu et al found that SVT occurred in 26% of patients. By age 10 years, >50% of RAI patients would have had >1 episode of SVT.16 In LAI patients, although bradyarrhythmia caused by either sinus node or atrioventricular node dysfunction is a common conduction disturbance,17 SVT remains a concern. Miyazaki et al found that supraventricular arrhythmia occurred in 23% of 83 LAI patients; although atrial arrhythmia was the most common arrhythmia mechanism, SVT and junctional ectopic tachycardia accounted for 19% of arrhythmia cases.18 In patients who have other CCHDs with single‐ventricle physiology, relevant studies about the electrophysiological characteristics were lacking, with only some case reports published.19, 20, 21
Tachycardia Related to TAVN in Patients With Heterotaxy and Other CCHDs
In the present study, the most common SVT mechanism in CCHD patients was TAVNRT, followed by AVRT, junctional tachycardia, and AVNRT. TAVN was previously suggested to be associated with RAI.9, 22, 23 On the basis of pathological findings, Dickinson et al suggested that paired atrioventricular nodes with a sling of conduction tissue are common in patients with heterotaxy and CCHDs.9 In the present study, the incidence of TAVN in RAI patients was as high as 72.2%, and TAVNRT accounted for 64.3% of SVT cases in the RAI patients. In addition, TAVNRT was not uncommon in the patients with LAI and other CCHDs. In the present study, 2 LAI patients with TAVN physiology shared similar cardiac anomalies including a common atrioventricular canal and double‐outlet right ventricle and pulmonary stenosis. In 3 patients with other CCHDs with TAVN, cardiac anomalies varied from crisscross atrioventricular valves to corrected transposition of the great arteries. Walsh et al presented 7 CCHD patients with TAVN. Two of these patients had RAI, and the remaining 5 had either a common atrioventricular canal or crisscross atrioventricular valves.10 These results suggest that TAVN is not unique in RAI patients and also can occur in patients with LAI and other CCHDs, particularly those with abnormal atrioventricular connection. Previous studies of catheter ablation of TAVNRT are very limited.10, 16 In the present study, both the success rate (10 of 12, 83.3%) and the recurrence rate (1 or 10, 10%) were acceptable. Consequently, catheter ablation was effective for complex SVT in CCHD patients.
Most of our patients with TAVN (77.8%) present with 1 dominant ECG pattern at resting ECG. The QRS morphology at surface ECG did not change after successful ablation of minor atrioventricular nodes in these patients. For the other 4 patients with fused QRS morphology at surface ECG, catheter ablation was performed in 3 patients and was successful in 2. The QRS duration was not prolonged after successful ablation in these 2 patients. Although cardiac synchronization might be a concern after ablation in these patients, in our limited experience, it did not cause dyssynchronization after successful ablation.
In addition to TAVNRT, another important arrhythmia mechanism related to TAVN is junctional tachycardia. Monckeberg was the first to identify sling conduction tissue between 2 atrioventricular nodes, which suggested that this tissue may be a reentry circuit.9, 24 Takeuchi et al further confirmed the presence of an intra‐Hisian sling through detailed mapping.25 Wu et al and Miyazaki et al17, 18 showed junctional tachycardia in certain LAI patients. In the present study, junctional tachycardia, which has narrow QRS tachycardia with dissociated VA conduction, was present in 3 patients—2 with RAI and 1 with LAI. In the RAI patients with junctional tachycardia, both had TAVN, and both could be induced by atrial extrastimulation; therefore, reentry through an intra‐Hisian sling may be the mechanism of this unusual type of junctional tachycardia. Catheter ablation was not further performed in these patients because of the high risk of complete atrioventricular block, which may aggravate hemodynamic deterioration in patients who have undergone Fontan surgery, even after pacemaker implantation.26 New ablation tools, such as the cryoablation technique, may be helpful because of their reversibility and can be valuable for treating this junctional tachycardia.27, 28 In addition, for CCHD with TAVNRT with nearby paired atrioventricular nodes, AVNRT without normal anatomical guidance, and accessory pathways close to a single atrioventricular node, cryoablation can also be useful.29
AVRT in CCHD Patients Was Often Associated With Multiple Accessory Pathways
AVRT is also a key mechanism of SVT in CCHD patients. In a large cohort study, Chetaille et al presented the outcome of catheter ablation in 83 patients with congenital heart disease. Multiple accessory pathways were present in 20% of patients, and the ablation success rate in patients without Ebstein's anomaly was 69%, with a recurrence rate of 19%. In their cohort, 17 patients had single‐ventricle CCHD, including 7 patients with heterotaxy syndrome30; however, that study did not further discuss those with single‐ventricle physiology. In the present study, AVRT accounted for 28.6% of SVT cases in the CCHD patients. Multiple accessory pathways, perhaps as many as 4, are very common and account for 50% of AVRT cases. Nevertheless, catheter ablation remains effective, with a success rate of 83.3% and a recurrence rate of 20% (1 in 5).
Vascular Access Problem in EPSs
Vascular access is crucial during EPS and catheter ablation in CCHD patients. Some patients are young when they undergo an EPS; some have vessel occlusion resulting from previous interventions, and some have completed Fontan surgery with only vascular access to the atrium as fenestration. In 3 of our patients, catheter ablation was not performed for these reasons. New mapping catheters, such as 2F multipolar catheters, have been shown to be helpful. We performed the EPS by using two 2F catheters from the internal jugular vein through a Glenn shunt to the pulmonary artery and to the right atrium and right ventricle in a patient with bilateral femoral vein occlusion (Figure 5). These small catheters can provide more precise mapping when vascular access is limited in CCHD patients.
Figure 5.
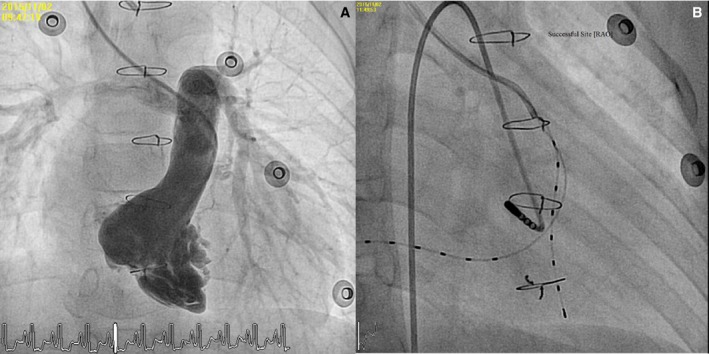
Electrophysiology study using limited catheter approach with small‐sized catheters in a patient with bilateral femoral vein occlusion. A 7‐year‐old boy with pulmonary atresia with intact ventricular septum receiving a right‐ventricular outflow tract patch and right‐site bidirectional Glenn shunt. He had total occlusion of the bilateral femoral vein resulting from previous interventions. A, Right ventriculogram showed a hypoplastic right ventricle with inlet and trabecular bipartile hypoplasia. B, We used a guiding catheter and two 2‐F multipolar catheters for right atrial and right ventricle signal sensing, pacing, and mapping. Because his accessory pathway was located at the left atrioventricular groove, we used a retrograde transaortic approach and successfully eliminated the accessory pathway. RAO indicates right anterior oblique view.
Conclusion
EPSs in 30 CCHD patients revealed that TAVN was common not only in the RAI patients but also in those with other single‐ventricle CCHDs. Transcatheter ablation of the minor atrioventricular node for TAVNRT, multiple accessory pathways for AVRT, and a slow pathway for AVNRT are effective approaches. Ablation feasibility is usually limited by vascular access and the risk of atrioventricular block, which may be overcome by using a new mapping catheter and ablation tools.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e004504 doi: 10.1161/JAHA.116.004504)
References
- 1. Gersony WM. Fontan operation after 3 decades: what we have learned. Circulation. 2008;117:13–15. [DOI] [PubMed] [Google Scholar]
- 2. Jaquiss R, Imamura M. Single ventricle physiology: surgical options, indications and outcomes. Curr Opin Cardiol. 2009;24:113–118. [DOI] [PubMed] [Google Scholar]
- 3. Diller GP, Giardini A, Dimopoulos K, Gargiulo G, Muller J, Derrick G, Giannakoulas G, Khambadkone S, Lammers AE, Picchio FM, Gatzoulis MA, Hager A. Predictors of morbidity and mortality in contemporary fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J. 2010;31:3073–3083. [DOI] [PubMed] [Google Scholar]
- 4. Reichlin A, Pretre R, Dave H, Hug MI, Gass M, Balmer C. Postoperative arrhythmia in patients with bidirectional cavopulmonary anastomosis. Eur J Cardiothorac Surg. 2014;45:620–624. [DOI] [PubMed] [Google Scholar]
- 5. Quinton E, Nightingale P, Hudsmith L, Thorne S, Marshall H, Clift P, de Bono J. Prevalence of atrial tachyarrhythmia in adults after Fontan operation. Heart. 2015;101:1672–1677. [DOI] [PubMed] [Google Scholar]
- 6. Ohuchi H, Miyazaki A, Watanabe T, Yamada O, Yagihara T, Echigo S. Hemodynamic deterioration during simulated supraventricular tachycardia in patients after the Fontan operation. Int J Cardiol. 2007;117:381–387. [DOI] [PubMed] [Google Scholar]
- 7. Blomstrom‐Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, Campbell WB, Haines DE, Kuck KH, Lerman BB, Miller DD, Shaeffer CW, Stevenson WG, Tomaselli GF, Antman EM, Smith SC Jr, Alpert JS, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Hiratzka LF, Hunt SA, Jacobs AK, Russell RO Jr, Priori SG, Blanc JJ, Budaj A, Burgos EF, Cowie M, Deckers JW, Garcia MA, Klein WW, Lekakis J, Lindahl B, Mazzotta G, Morais JC, Oto A, Smiseth O, Trappe HJ; European Society of Cardiology Committee N‐HRS . ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias–executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias) developed in collaboration with NASPE‐Heart Rhythm Society. J Am Coll Cardiol. 2003;42:1493–1531. [DOI] [PubMed] [Google Scholar]
- 8. Wu MH, Lin JL, Wang JK, Chiu IS, Young ML. Electrophysiological properties of dual atrioventricular nodes in patients with right atrial isomerism. Br Heart J. 1995;74:553–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dickinson DF, Wilkinson JL, Anderson KR, Smith A, Ho SY, Anderson RH. The cardiac conduction system in situs ambiguus. Circulation. 1979;59:879–885. [DOI] [PubMed] [Google Scholar]
- 10. Epstein MR, Saul JP, Weindling SN, Triedman JK, Walsh EP. Atrioventricular reciprocating tachycardia involving twin atrioventricular nodes in patients with complex congenital heart disease. J Cardiovasc Electrophysiol. 2001;12:671–679. [DOI] [PubMed] [Google Scholar]
- 11. Bae EJ, Noh CI, Choi JY, Yun YS, Kim WH, Lee JR, Kim YJ. Late occurrence of adenosine‐sensitive focal junctional tachycardia in complex congenital heart disease. J Interv Card Electrophysiol. 2005;12:115–122. [DOI] [PubMed] [Google Scholar]
- 12. Gewillig M. The Fontan circulation. Heart. 2005;91:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiu SN, Lu CW, Chang CW, Chang CC, Lin MT, Lin JL, Chen CA, Wang JK, Wu MH. Radiofrequency catheter ablation of supraventricular tachycardia in infants and toddlers. Circ J. 2009;73:1717–1721. [DOI] [PubMed] [Google Scholar]
- 14. Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, Dahl SH, Cannon BC, O'Leary PW, Driscoll DJ, Cetta F. 40‐year follow‐up after the Fontan operation: long‐term outcomes of 1,052 patients. J Am Coll Cardiol. 2015;66:1700–1710. [DOI] [PubMed] [Google Scholar]
- 15. Loomba RS, Willes RJ, Kovach JR, Anderson RH. Chronic arrhythmias in the setting of heterotaxy: differences between right and left isomerism. Congenit Heart Dis. 2016;11:7–18. [DOI] [PubMed] [Google Scholar]
- 16. Wu MH, Wang JK, Lin JL, Lin MT, Chiu SN, Chen CA. Long‐term outcome of twin atrioventricular node and supraventricular tachycardia in patients with right isomerism of the atrial appendage. Heart Rhythm. 2008;5:224–229. [DOI] [PubMed] [Google Scholar]
- 17. Wu MH, Wang JK, Lin JL, Lai LP, Lue HC, Hsieh FJ. Cardiac rhythm disturbances in patients with left atrial isomerism. Pacing Clin Electrophysiol. 2001;24:1631–1638. [DOI] [PubMed] [Google Scholar]
- 18. Miyazaki A, Sakaguchi H, Ohuchi H, Yamamoto T, Igarashi T, Negishi J, Toyota N, Kagisaki K, Yagihara T, Yamada O. The incidence and characteristics of supraventricular tachycardia in left atrial isomerism: a high incidence of atrial fibrillation in young patients. Int J Cardiol. 2013;166:375–380. [DOI] [PubMed] [Google Scholar]
- 19. Ajiki K, Hayami N, Kasaoka Y, Imai Y, Fujiu K, Murakawa Y. Supraventricular tachycardia originating from the posterior atrioventricular node in the univentricular heart with single atrium. Int Heart J. 2007;48:253–259. [DOI] [PubMed] [Google Scholar]
- 20. Pedrote A, Arana E, Garcia‐Riesco L, Maya E, Errazquin F. Radiofrequency catheter ablation of an orthodromic tachycardia in a patient with single ventricle. Int J Cardiol. 2006;112:e38–e39. [DOI] [PubMed] [Google Scholar]
- 21. Yalta K, Yontar C, Karadas F, Yilmaz MB, Turgut OO, Yilmaz A, Erdem A, Tandogan I. Initial syncope associated with alternating attacks of supraventricular tachycardia and atrioventricular block long after surgical correction of tricuspid atresia. Cardiol J. 2008;15:186–188. [PubMed] [Google Scholar]
- 22. Wu MH, Wang JK, Lin JL, Lai LP, Lue HC, Young ML, Hsieh FJ. Supraventricular tachycardia in patients with right atrial isomerism. J Am Coll Cardiol. 1998;32:773–779. [DOI] [PubMed] [Google Scholar]
- 23. Bae EJ, Noh CI, Choi JY, Yun YS, Kim WH, Lee JR, Kim YJ. Twin AV node and induced supraventricular tachycardia in Fontan palliation patients. Pacing Clin Electrophysiol. 2005;28:126–134. [DOI] [PubMed] [Google Scholar]
- 24. Monckeberg JG. Zur entwicklungsgeschichte der atrio ventrickular systems. Verhandl Deutsch Path Gesellsch. 1913;16:228. [Google Scholar]
- 25. Takeuchi D, Shoda M, Takahashi K, Naknishi T. Absence of a resetting phenomenon suggests that a sling works as a part of the supraventricular tachycardia circuit involving twin atrioventricular nodes: a case of corrected transposition of the great arteries. HeartRhythm Case Rep. 2015;1:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams RV, Travison T, Kaltman JR, Cecchin F, Colan SD, Idriss SF, Lu M, Margossian R, Reed JH, Silver ES, Stephenson EA, Vetter VL. Comparison of Fontan survivors with and without pacemakers: a report from the Pediatric Heart Network Fontan Cross‐Sectional Study. Congenit Heart Dis. 2013;8:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Law IH, Von Bergen NH, Gingerich JC, Saarel EV, Fischbach PS, Dick M II. Transcatheter cryothermal ablation of junctional ectopic tachycardia in the normal heart. Heart Rhythm. 2006;3:903–907. [DOI] [PubMed] [Google Scholar]
- 28. Collins KK, Van Hare GF, Kertesz NJ, Law IH, Bar‐Cohen Y, Dubin AM, Etheridge SP, Berul CI, Avari JN, Tuzcu V, Sreeram N, Schaffer MS, Fournier A, Sanatani S, Snyder CS, Smith RT Jr, Arabia L, Hamilton R, Chun T, Liberman L, Kakavand B, Paul T, Tanel RE. Pediatric nonpost‐operative junctional ectopic tachycardia medical management and interventional therapies. J Am Coll Cardiol. 2009;53:690–697. [DOI] [PubMed] [Google Scholar]
- 29. Collins KK, Schaffer MS. Use of cryoablation for treatment of tachyarrhythmias in 2010: survey of current practices of pediatric electrophysiologists. Pacing Clin Electrophysiol. 2011;34:304–308. [DOI] [PubMed] [Google Scholar]
- 30. Chetaille P, Walsh EP, Triedman JK. Outcomes of radiofrequency catheter ablation of atrioventricular reciprocating tachycardia in patients with congenital heart disease. Heart Rhythm. 2004;1:168–173. [DOI] [PubMed] [Google Scholar]


