Abstract
Background
Malondialdehyde (MDA) is generated during lipid peroxidation as in oxidized low‐density lipoprotein, but antibodies against oxidized low‐density lipoprotein show variable results in clinical studies. We therefore studied the risk of cardiovascular disease (CVD) associated with IgM antibodies against MDA conjugated with human albumin (anti‐MDA).
Methods and Results
In a 5‐ to 7‐year follow‐up of 60‐year‐old men and women from Stockholm County previously screened for cardiovascular risk factors (2039 men, 2193 women), 209 incident CVD cases (defined as new events of coronary heart disease, fatal and nonfatal myocardial infarction, ischemic stroke, and hospitalization for angina pectoris) and 620 age‐ and sex‐matched controls were tested for IgM anti‐MDA by ELISA. Antibody peptide/protein characterization was done using a proteomics de novo sequencing approach. After adjustment for smoking, body‐mass index, type 2 diabetes mellitus, hyperlipidemia, and hypertension, an increased CVD risk was observed in the low IgM anti‐MDA percentiles (below 10th and 25th) (odds ratio and 95% CI: 2.0; 1.19–3.36 and 1.67; 1.16–2.41, respectively). Anti‐MDA above the 66th percentile was associated with a decreased CVD risk (odds ratio 0.68; CI: 0.48–0.98). After stratification by sex, associations were only present among men. IgM anti‐MDA levels were lower among cases (median [interquartile range]: 141.0 [112.7–164.3] versus 147.4 [123.5–169.6]; P=0.0177), even more so among men (130.6 [107.7–155.3] versus 143.0 [120.1–165.2]; P=0.001). The IgM anti‐MDA variable region profiles are distinctly different and also more homologous in their content (correlates strongly with fewer peptides) than control antibodies (not binding MDA).
Conclusions
IgM anti‐MDA is a protection marker for CVD. This finding could have diagnostic and therapeutic implications.
Keywords: antibody, cardiovascular disease, cardiovascular disease risk factors, immune system, malondialdehyde, oxidation, proteomics
Subject Categories: Biomarkers, Basic Science Research, Mechanisms, Clinical Studies, Cardiovascular Disease
Introduction
Cardiovascular disease (CVD) is a leading cause of death and morbidity worldwide. The main underlying cause of CVD is atherosclerosis, which is characterized by activated immune‐competent cells, foam cells filled with oxidized low‐density lipoprotein (OxLDL), and dead cells in the arterial atherosclerotic lesions.1, 2 An important role of the immune system in atherosclerosis is implicated by pivotal studies in animal models demonstrating that immunization can influence atherosclerosis. For example, OxLDL decreased3 while heat shock proteins 60/65 increased atherosclerosis when used as antigens for immunization.4
It has been described earlier that malondialdehyde (MDA)‐modified LDL is scavenged by monocytes/macrophages in atherosclerotic plaques and thus could play a role in atherogenesis.5 One dominant epitope in MDA‐protein adducts is acetaldehyde, which forms stable dihydropyridine (4‐methyl‐1,4‐dihydropyridine‐3,5‐dicarbaldehyde), which in turn modifies ubiquitous and essential amino acid lysine to a stable product that is implicated in atherosclerosis and other inflammatory conditions.6
There is a need for novel CVD risk and protection markers, especially factors that account for aspects of inflammation and immunity, since traditional risk factors such as age, male sex, hypertension, hyperlipidemia, smoking, and diabetes mellitus do not account for this. Among emerging and/or nontraditional risk markers, high‐sensitivity C‐reactive protein (hsCRP) and IL‐6, which both exhibit high sensitivity, have been discussed intensively.7 However, the volatility of these measures may pose a limitation for clinical usefulness at the individual level.2, 8, 9 Other emerging risk markers include OxLDL measures10 and LDL‐PLA2.11
Among immunological risk markers, several autoantibodies have been studied in many reports, especially against various epitopes from oxidized LDL (including MDA, apoB100, and those arising from copper ion oxidation). Currently the evidence is conflicting, with antibodies against such compounds being described as both risk and protection markers in studies performed.2 We have demonstrated that antibodies against phosphorylcholine conjugated with albumin are negatively associated with atherosclerosis development, CVD, and also autoimmune disease.2 We focus here on the MDA epitope, conjugated with human serum albumin and not with OxLDL‐related carriers.
We studied the role of IgM anti‐MDA in a large prospective cardiovascular study, the cohort of 60‐year‐old men and women from Stockholm (60YO) and characterize the antibodies. The implications of the findings are discussed.
Methods
Subjects
The 60YO is a large prospective cardiovascular study described in detail elsewhere.12 Briefly, from July 1, 1997 to June 30, 1998, every third man and woman living in a part of the County of Stockholm, Sweden, reaching the age of 60 years, was invited to participate in a health screening for CVDs. In total, 4232 subjects (2039 men and 2193 women; response rate 78%) participated in the study. Information on sociodemography, lifestyle habits, medication, and previous diseases and hospitalizations was obtained by a self‐administered questionnaire. Physical examination including blood pressure measurements, anthropometry, and ECG was performed. Serum, plasma, and whole blood were collected for storage in a biobank (−80°C). Details of the screening procedure have been described elsewhere.12 The study was approved by the Karolinska Institutet research ethics committee and is in accordance with the Declaration of Helsinki. All subjects gave informed consent before entering the study.
A Nested Case–Control Design
To record incident cases of first CVD, new events of coronary heart disease, defined as fatal and nonfatal myocardial infarction (MI) and ischemic stroke and hospitalization for angina pectoris, were registered. The study base of 4232 subjects was matched with the national cause‐of‐death registry (fatal events until December 31, 2003) and the national in‐hospital registry (nonfatal events until December 31, 2005). Through these matching procedures, 211 incident cases of CVD were recorded. Only living subjects without a history of CVD prior to recruitment were included in the matching procedures. The International Classification of Diseases (ICD‐10) was used to register coronary heart disease deaths (I 20, I 21, I 46), MI (I 21), angina pectoris including percutaneous coronary interventions and coronary artery bypass grafts (I 20, Z 95.5 and Z 95.1), and ischemic stroke (I 63–I 66). For each case, 3 controls were randomly selected, matched for sex and age (±60 days). Thus, a nested case–control design (211 cases and 633 controls) was applied for the epidemiological and statistical analyses and 209 cases and 620 controls were available for testing of the IgM anti‐MDA levels.
Determination of IgM Antibodies Against MDA With ELISA
IgM antibodies to MDA were determined by ELISA essentially as described.13 Serum from a donor with an anti‐MDA level above median level was used as internal standard and tested on every plate. The plateau of antibody binding was reached with the antigen concentration of 10 μg/mL. Nunc Immuno microwell plates (Thermo Labsystems, Franklin, MA) were coated with MDA (conjugated with human serum albumin as described above). Coated plates were incubated overnight at 4°C. After 5 washings with PBS, the plates were blocked with 2% BSA‐PBS for 90 minutes at room temperature and washed as described above. Serum samples were diluted (1:200) in 0.2% BSA‐PBS and added at 50 μL/well.
Plates were incubated at room temperature for 2 hours and washed as described above. Biotin‐conjugated goat anti‐human IgM (diluted 1:22 000 in the 1% BSA) was added at 100 μL/well and incubated at room temperature for 2 hours. After 4 washings, the plate was incubated with horseradish peroxidase conjugated streptavidin (diluted 1:6000) (Thermo Scientific, Denmark) at 100 μL/well for 20 minutes. The color was developed by adding the horseradish peroxidase substrate, TMB (3,3′,5,5′‐tetramethylbenzidine; Sigma Aldrich, MO), at 100 μL/well and incubating the plates for 8 minutes at room temperature in the dark. Further reaction was stopped with stop solution (Sigma Aldrich, MO) at 50 μL/well. Finally, plates were read in an ELISA Multiscan Plus spectrophotometer (Spectra Max 250; Molecular Devices, CA) at 450 nm. All samples were measured in duplicate within a single assay and the coefficient of variation between the duplicates was below 15%.
In order to investigate the specificity of IgM anti‐MDA, competition assays were performed. At a dilution giving 50% of maximal binding to anti‐MDA, sera were preincubated with different concentrations of MDA overnight in glass tubes. After vortexing, the tubes were incubated at 4°C overnight and centrifuged at 15100 g for 30 minutes (4°C). The supernatants were tested for antibody binding to MDA as described. The method demonstrated competition above 60% (data not shown), similar to the method we described previously.13, 14 The percentage of inhibition was calculated as follows:
Extraction of Anti‐MDA From Human IgM
IgM anti‐MDA was extracted according to previously described protocol.13 In short, MDA–human serum albumin, 1.5 mg/mL, was coupled to a HiTrap NHS column (GE Healthcare, Sweden).
Human IgM (Sigma Aldrich, Israel) was passed through an MDA–human serum albumin Sepharose column. Unbound IgM considered as non‐anti‐MDA (flow through, FT) was collected by washing the columns with binding buffer. After washing steps with binding buffers, bound anti‐MDA‐IgM was eluted with 0.1 mol/L glycine–HCl elution buffer. Further eluted antibodies were desalted in PD‐10 columns (GE Healthcare, UK) and concentrated by a Centriprep centrifugal filter (Millipore, Ireland). After filtration through a 0.22‐μm filter (Sarstedt, Germany), extracted antibodies were stored at −20°C.
Anti‐MDA and Non‐Anti‐MDA IgM Protein and Peptide Characterization
Samples from 3 anti‐MDA extractions were digested in duplicates at 2 occasions and run individually and in pools. The results (ie, n=6/sample) were then combined and statistically compared according to the original anti‐MDA (n=3) and FT (non‐anti‐MDA, n=3) extractions. Anti‐MDA and non‐anti‐MDA (FT) IgM samples (10 μg) were reduced with 20 mmol/L dithiothreitol for 30 minutes at 56°C and alkylated with 66 mmol/L iodoacetamide for 30 minutes in the dark. Trypsin was added at a ratio of 1:30 (enzyme:protein) and the proteins were digested at 37°C overnight. Tryptic peptides were desalted using C18 StageTips (Thermo Scientific), dried in a SpeedVac, and resuspended in 0.1% formic acid and 1% acetonitrile. As previously described,15 samples were injected onto a reversed‐phase 15‐cm column (PepMap, C18, 3 μm, 100 Å) in 1‐μg aliquots using a nano–liquid chromatography system Ultimate 3000 connected to a Fusion Orbitrap mass spectrometer (both—Thermo Fisher Scientific). Briefly, survey mass spectra were acquired in the range of m/z 300 to 1700 with a nominal resolution of 120 000. Precursor ion selection for high‐energy collision dissociation and electron‐transfer dissociation fragmentation was performed in the “top speed” mode on monoisotopic ions with the most intense precursor priority and with a minimum intensity of 50 000. Prior to de novo sequencing, MS/MS spectra were first searched against a human reference proteome (February 2014, 89 027 UniProt protein sequences). Morpheus (v.165) was used as a search engine, applying the criteria: up to 2 missed tryptic cleavages, 10 and 20 ppm mass tolerances for precursor and fragment peaks, respectively, carbamidomethylation of cysteine as fixed modification, and oxidation of methionine, deamidation of asparagine and glutamine as well as acetylation of protein N‐terminus as variable modifications. Peptide sequences with a <1% false discovery rate were excluded. The remaining data underwent de novo sequencing using pNovo+ (v.1.3)15 via a limited precursor mass range of 700 to 4000 Da, oxidized methionine as an independent residue, and mass tolerance set at 5 ppm for precursors and 15 ppm for fragments. Up to 9 top sequence candidates were generated for each high‐energy collision dissociation‐electron‐transfer dissociation MS/MS pair. These candidates were homology‐searched against the UniProt protein database using BLASTp. Since leucine (Leu/L) and isoleucine (Iso/I) were difficult to distinguish in de novo sequencing, all isoleucine residues (I) in the protein sequence database were converted to leucine (L). The match with the highest BLAST score was reported as the final sequence for a given high‐energy collision dissociation‐electron‐transfer dissociation spectral pair. Raw mass spectrometry data were processed through the DeMix‐Q workflow16, 17 in which MS/MS spectra were matched against the database combining the de novo sequenced and known peptides and using the Morpheus search engine with the same parameters as described above. Peptide abundances were reported as the summed integrals of ion currents from all charge states. Assignment of de novo sequenced peptides to complement determining regions (CDR) and framework regions were based on Uniprot information and by using the VBASE sequence directory (Tomlinson et al, MRC Centre for Protein Engineering, http://www2.mrc-lmb.cam.ac.uk/vbase/alignments2.php).
The abundances of IgM peptides were normalized so that the total abundance was the same (100%) in all samples.
Statistical Analysis
Various data analyses including demographic biochemistry‐ and anthropometry‐related were performed for cases and controls, respectively, with values expressed as mean±SD for normally distributed parameters and medians (ranges) for parameters that were not normally distributed after logarithmic transformation. Statistical differences between cases and controls were evaluated through parametric tests. Odds ratios (OR) with 95% CI were calculated applying conditional logistic regression with anti‐MDA levels divided into 7 percentiles as indicated. For the analyses of specific percentiles, the remaining values formed the reference. Analyses were run crude or adjusted for traditional risk factors as indicated. These analyses were performed using SAS 9.4 release (SAS Institute, Cary, NC).
Differences between anti‐MDA and non‐anti‐MDA IgM peptides were tested using 2‐tailed Student t test with equal or unequal variance depending upon F‐test. For all statistical analyses, a P<0.05 was considered significant. Principal component analysis and Orthogonal Projections to Latent Structures Discriminate Analysis were performed using SIMCA 14.0 (Umetrics, Umeå, Sweden) following mean centering, log scaling, and unit variance scaling.
Results
Clinical Associations
We identified 211 incident cases of first CVD events throughout the follow‐up period (77 with MI, 85 with angina pectoris, and 49 with ischemic stroke). For each incident case, 3 age‐ and sex‐matched controls were selected (633 controls in total). Serum samples were missing for 2 cases and 13 controls, leaving 209 cases and 620 controls for analyses.
As previously reported in a similar dataset,18 there were more hypertensives and smokers among the cases than controls and a trendwise higher body mass index. Blood pressure level, high‐density lipoprotein, and high‐sensitivity C‐reactive protein were associated with risk among cases as compared to controls (Table 1).
Table 1.
Baseline Characteristics Among Incident CVD Cases and Matched Controls
| Incident Cases | Controls | P Value | |
|---|---|---|---|
| Number | 209 | 620 | NA |
| Age, y | 60 | 60 | NA |
| Male sex, % | 66.0 | 66.8 | NA |
| Smokers, % | 32.0 | 19.7 | 0.0002 |
| Diabetes mellitus, % | 24.4 | 15.7 | 0.0042 |
| BMI, kg/m2 | 27.8±4.6 | 26.6±3.8 | 0.0030 |
| Hypertension (>140/90 mm Hg), % | 42.6 | 25.7 | <0.0001 |
| Glucose, mmol/L | 6.1±2.5 | 5.6±1.5 | 0.0004 |
| Insulin, μmol/L | 11.4±7.1 | 10.1±59 | 0.0140 |
| Systolic blood pressure, mm Hg | 148±21.8 | 139±21.2 | <0.0001 |
| Diastolic blood pressure, mm Hg | 98±10.6 | 85±10.4 | <0.0001 |
| Cholesterol, mmol/L | 6.1±1.0 | 6.0±1.2 | 0.1366 |
| HDL, mmol/L | 1.3±0.4 | 1.4±0.4 | 0.0006 |
| LDL, mmol/L | 3.9±1.2 | 3.8±1.1 | 0.4490 |
| Triglycerides, mmol/L | 1.6±1.0 | 1.4±0.8 | 0.0003 |
| hsCRP, mg/L | 2.4 (1.3–4.6) | 1.7 (0.9–3.2) | <0.0001 |
| Anti‐MDA IgM units | 141.0 (112.7–164.3) | 147.4 (123.5–169.6) | 0.0177 |
| Anti‐MDA IgM units men | 130.6 (107.7–155.3) | 143.0 (120.1–165.2) | 0.0010 |
| Anti‐MDA IgM units women | 154.0 (133.7–187.6) | 155.1 (134.7–176.7) | 0.5638 |
Data are presented as percentage, mean±SD, or median with interquartile ranges within parentheses. BMI indicates body mass index; CVD, cardiovascular disease; HDL, high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; MDA, malondialdehyde.
IgM Anti‐MDA levels were lower among cases (median [interquartile range]: 141.0 [112.7–164.3] versus 147.4 [123.5–169.6]; P=0.0177). These associations were stronger when only men were included in the analysis: (130.6 [107.7–155.3] versus 143.0 [120.1–165.2]; P=0.001). IgM anti‐MDA levels were divided in percentiles and low or high levels were compared with the rest as indicated (Table 2). After adjustment for smoking, body mass index, type 2 diabetes mellitus, hypercholesterolemia, and hypertension, an increased risk was observed in the low percentiles of IgM anti‐MDA, which was significant at the 10th percentile: OR 2.00, CI (1.19–3.36) and 25th: OR 1.67, CI (1.16–2.41). For values IgM anti‐MDA values above the 66th percentile, as compared to lower percentiles, OR was 0.68, CI (0.48–0.98).
Table 2.
Association Between Levels of IgM Anti‐MDA and Risk for MI and/or+Stroke (CVD), Among All Participants and Men and Women Separately
| Anti‐MDAa | All | Males | Females | |||
|---|---|---|---|---|---|---|
| Crude | Adjustedb | Crude | Adjusteda | Crude | Adjusteda | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| ≤10% | 2.16 (1.31–3.55) | 2.00 (1.19–3.36) | 2.62 (1.48–4.66) | 2.42 (1.32–4.42) | 1.14 (0.39–3.37) | 1.08 (0.36–3.29) |
| ≤25% | 1.76 (1.24–2.52) | 1.67 (1.16–2.41) | 1.98 (1.30–3.01) | 1.94 (1.25–3.01) | 1.32 (0.66–2.62) | 1.15 (0.57–2.31) |
| ≤33% | 1.48 (1.06–2.06) | 1.40 (0.99–1.97) | 1.79 (1.20–2.68) | 1.75 (1.15–2.68) | 0.94 (0.50–1.78) | 0.83 (0.43–1.62) |
| >50% | 0.82 (0.59–1.12) | 0.83 (0.59–1.16) | 0.71 (0.48–1.05) | 0.70 (0.46–1.06) | 1.10 (0.62–1.94) | 1.11 (0.61–2.01) |
| >66% | 0.68 (0.48–0.97) | 0.68 (0.48–0.98) | 0.54 (0.34–0.86) | 0.54 (0.33–0.86) | 0.99 (0.56–1.73) | 0.96 (0.54–1.73) |
| >75% | 0.84 (0.57–1.23) | 0.80 (0.53–1.19) | 0.59 (0.34–1.02) | 0.54 (0.31–0.96) | 1.29 (0.73–2.29) | 1.28 (0.70–2.36) |
| >90% | 0.85 (0.48–1.49) | 0.82 (0.46–1.46) | 0.34 (0.12–0.97) | 0.32 (0.11–0.98) | 1.62 (0.77–3.42) | 1.47 (0.68–3.19) |
Analyses are done by use of conditional logistic regression. CVD indicates cardiovascular disease; MDA, malondialdehyde; MI, myocardial infarction; OR, odds ratio.
For each percentile used as cut‐off, the remaining values formed the reference.
Adjustment for smoking, body mass index, type 2 diabetes mellitus, hypercholesterolemia, and hypertension.
The corresponding OR and CI in the multivariate analysis for smoking, body mass index, type 2 diabetes mellitus, hypercholesterolemia, and hypertension were calculated when different anti‐MDA levels were compared. In general, only smoking and hypertension were significant in the model. In the low percentiles of IgM anti‐MDA at the 10th percentile, OR and CI for smoking was 1.92 and 1.32 to 2.79 and for hypertension 1.96 and 1.36 to 2.71. At the 25th percentile, OR and CI for smoking was 1.93 and 1.32 to 2.80 and for hypertension 1.96 and 1.38 to 2.77. For IgM anti‐phosphorylcholine (PC) values above 66th percentile, OR and CI for smoking was 1.94 and 1.33 to 2.80 and for hypertension 1.99 and 1.40 to 2.82.
For men these associations were even more pronounced, while no association was observed in women. Among men, at low levels we observed an increased CVD risk: below 10th percentile: OR 2.42, CI (1.32–4.42); 25th: OR 1.94, CI (1.25–3.01); 33rd: OR 1.75, CI (1.15–2.68).
Above the median, the protection effect was significant: above 66th percentile: OR 0.54, CI (0.33–0.86); above 75th percentile: OR 0.54, CI (0.33–0.96); and above 90th percentile: OR 0.32, CI (0.11–0.98). OR and CI for smoking, body mass index, type 2 diabetes mellitus, hypercholesterolemia, and hypertension when men were studied were comparable to the values presented above for the whole cohort, at different percentiles. In the low percentiles of IgM, anti‐MDA at the 10th percentile OR and CI for smoking was 2.04 and 1.26 to 3.30 and for hypertension 1.75 and 1.15 to 2.66. At the 25th percentile, OR and CI for smoking was 2.07 and 1.28 to 3.35 and for hypertension 1.82 and 1.20 to 2.78. For IgM anti‐PC values above 66th percentile, OR and CI for smoking was 2.10 and 1.30 to 3.41 and for hypertension 1.80 and 1.18 to 2.74. Among women, only hypertension reached statistical significance in the multivariate model (data not shown).
In general, similar associations were noted when we did not control for confounders (Table 2). When divided into stroke or MI/angina, associations did not reach statistical significance for stroke, where there were only 46 cases; still, OR at high levels of anti‐MDA was low, above 66th percentile OR 0.27, CI 0.07 to 1.04 and above the highest 75th percentile—OR 0.20, CI 0.04 to 1.03.
When restricting the outcome to MI/angina, in the whole group, values below the 10th and 25th percentile, respectively, were associated with increased risk: OR 1.70, CI 1.11 to 2.60 and OR 2.19, CI 1.23 to 3.91, respectively. Significant associations were absent among women, but were found when men were analyzed separately. At low levels, below 10th, 25th, and 33rd percentile, the risk was increased: OR 2.73, CI 1.36 to 5.46, OR 1.98, CI 1.20 to 3.29, and OR 1.82, CI 1.12 to 2.94, respectively. High levels, above 66th percentile, were associated with a significantly decreased risk: OR 0.58, CI 0.34 to 0.97.
Characteristics of Polyclonal Anti‐MDA IgM Variable Region
In total, 2429 peptide sequences were identified in quantifiable amounts in the LC‐MS/MS peptide sequencing experiments (Figure 1). Of these peptides, 1061 were derived via de novo sequencing and 738 showed distinct sequence homology with the variable regions. It is noteworthy that approximately only 20% of all characterized peptides were found in the anti‐MDA samples compared to the non‐anti‐MDA FT (Figure 1). One likely reason for this is that the anti‐MDA IgM are more homologous among themselves compared to the FT. However, it was also observed that the anti‐MDA samples generally contained less IgM conserved chain peptides. Thus, to adjust for concentration differences, we normalized the peptide abundances in samples to peptides that were identified in anti‐MDA samples, with or without their identification in FT. Furthermore, of these peptides we herein focus on the peptides that showed sequence homology to the IgM variable regions (n=147). Note that if the complete FT variable region profile is included (n=738), the results (comparing anti‐MDA and FT) will be similar, with an increased difference between the IgM pools (Figure 1).
Figure 1.
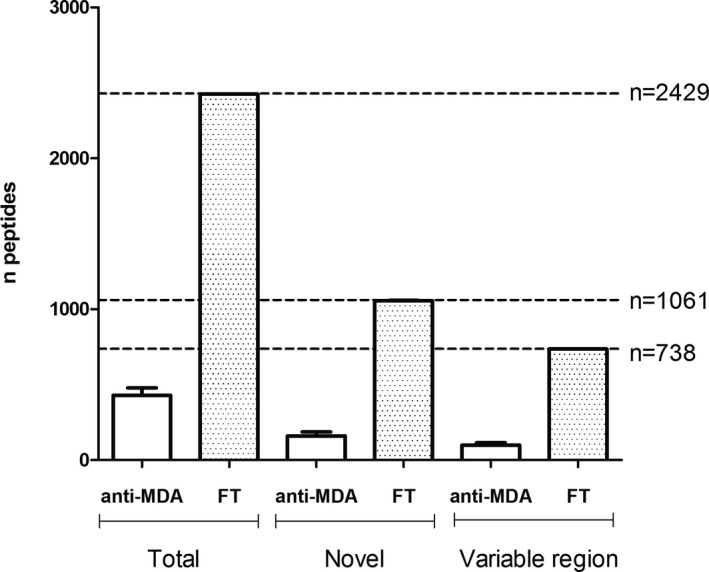
Numbers of peptides quantified in the anti‐MDA and in the flow through (FT) control samples. MDA indicates malondialdehyde.
Overall, fewer lambda chains were identified in the anti‐MDA samples. In comparison of the distributions of identified lambda variable (LV), kappa variable (KV), and heavy variable (HV) chains, the distribution of LV (lower, P=0.03) and KV (higher, P=0.03) chains were significantly different in anti‐MDA compared to FT (Figure 2A). Interestingly, one HV CDR3 peptide sequence (identified via peptides ENDNKFSFDYWGQGTLVTVSSASTK and FSFDYWGQGTLVTVSSASTK), and one KV CDR2 peptide sequence (identified via peptides ASTLQSGVPSR and LLLYAASTLQSGVPSR) were significantly elevated (P=0.01 and 0.001, respectively) in the anti‐MDA samples (Figure 2B). Additionally, heavy and kappa chain region sequences were also elevated in the anti‐MDA IgM profile, particular the framework sequences (Figure 2B). The differences between the anti‐MDA and FT HV‐, KV‐, and LV‐chain profiles (n=147) were further confirmed by multivariate statistics. The Principal component analysis model distinctly separated anti‐MDA and FT along the first component t[1], (R 2=0.59, Q2=0.37), which signifies that the anti‐MDA and FT variable region profiles are indeed distinctly different (Figure 3A). Moreover, when extracting out the peptides that were most prominently contributing to these differences (using Latent Structures Discriminate Analysis‐DA modeling with 95% CI, Figure 3B), it is evident that the anti‐MDA profile is more homologous in its content (ie, correlates strongly with fewer peptides, n=11) compared to the heterogeneous FT, which is correlating with high confidence with a majority of the rest of the peptides (n=71). Note that in reality the FT samples are even more heterogeneous since the MVA analysis only included the peptides that were found in the anti‐MDA and excluded the ≈600 additional variable region peptides that were only found in the FT.
Figure 2.
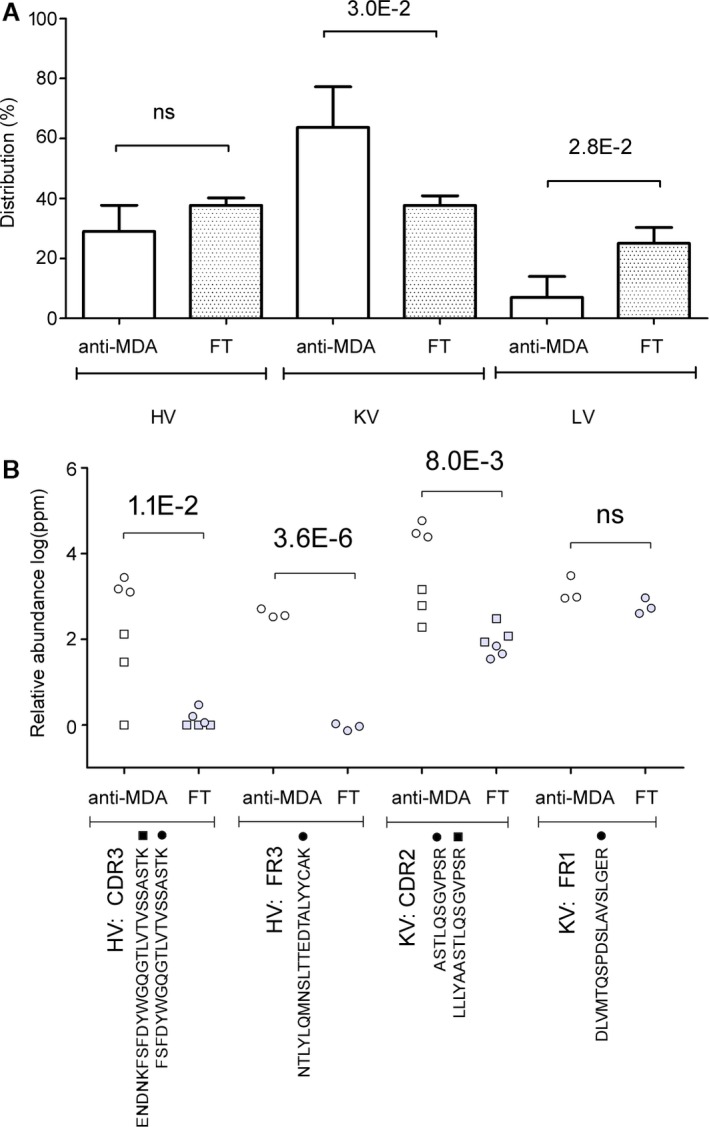
Differences in the variable chain region between polyclonal anti‐MDA IgM and non‐anti‐MDA IgM (flow through, FT). A, Distribution in heavy variable (HV), kappa variable (KV), and lambda variable (LV) chains in the anti‐MDA and non‐anti‐MDA FT samples. B, Peptides from the HV and KV regions that were elevated in the anti‐MDA IgM. Numbers indicate significant P‐values. CDR indicates complementary determining region; FR, framework region; ns, not significant. MDA indicates malondialdehyde.
Figure 3.
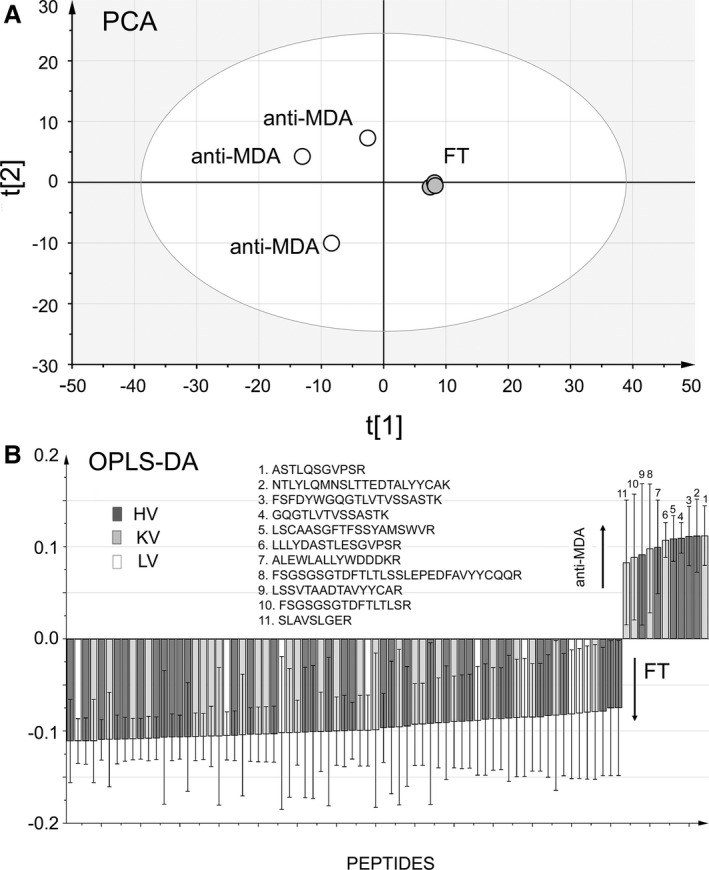
Multivariate analysis of the anti‐MDA and flow through (FT) samples using heavy variable, lambda variable, and kappa variable chain peptides that were identified in both FT and anti‐MDA samples or identified in anti‐MDA only. A, Principal component analysis (PCA), scores plot. The anti‐MDA and FT samples are distinctly separated along component 1 (t[1], R 2=0.59, Q2=0.37). B, Orthogonal Projections to Latent Structures–Discriminant Analysis (OPLS‐DA) loading plot. The plot shows which peptides are most distinctly different between the 2 IgM profile types (ie, anti‐MDA and FT). Only peptides correlating with 95% CI with respective sample type are shown. From the plot it is evident that the majority of peptides correlate with the FT, but a small number of specific peptides are correlating with the anti‐MDA IgM. It is noteworthy that no lambda chain sequences correlated with anti‐MDA. Heavy variable (HV), kappa variable (KV), and lambda variable (LV) sequences are shown. MDA indicates malondialdehyde.
Discussion
We here report that IgM antibodies against MDA bound to human albumin are significantly decreased in the 60‐year‐olds who develop CVD within a 5‐year follow‐up. Furthermore, having low IgM anti‐MDA levels (below 10th percentile) was associated with a 2‐fold increased risk of CVD within 5 years, which was also the case at the lowest 25th percentile. Levels of IgM anti‐MDA above the 66th percentile were associated with a reduced risk of CVD. These associations were independent of other traditional risk markers.
Interestingly, when women and men were analyzed separately, it became apparent that the properties of IgM anti‐MDA as a protection marker were only present in men. Those in the lowest decile had a 2.5‐fold increased risk and those in the highest had a 3‐fold protection. There were fewer women among the cases, which is in line with the lower age‐adjusted risk for women to develop CVD, which may result in insufficient power to detect an association.
Modifications of LDL, leading to generation of different epitopes simultaneously, involve oxidation or enzymatic changes by somewhat different methods that all could be relevant for atherosclerosis, where lipid peroxidation and production of inflammatory factors as lysophosphatidylcholine.2
In general, studies of antibodies against different forms of OxLDL and their role in atherosclerosis and CVD are conflicting, and anti‐OxLDL‐related antibodies have been described as either risk or protection markers or not being markers associated with CVD at all.2
In the first published study on anti‐OxLDL in the CVD field where MDA‐modified LDL was used, a positive independent association with atherosclerosis development was reported.19 In other previous studies, anti‐OxLDL was associated with peripheral vascular disease,20 development of MI,21, 22 and angiographically verified coronary artery disease.23 These findings were in line with animal studies, where strongly increased anti‐OxLDL levels in LDL receptor‐deficient mice with increased atherosclerosis were reported.24
In other cross‐sectional studies from the 1990s, neither significant association with coronary stenosis or CVD,25, 26 nor prediction of CVD longitudinally27 were noted.
We reported for the first time, using 2 different methods, a negative association in CVD with borderline hypertension, thus implicating anti‐OxLDL as protection markers in humans.28 This finding is more in line with important animal experiments where (MDA‐modified) OxLDL was associated with ameliorated atherosclerosis development,3 a result confirmed in several subsequent studies.2
In line with this, anti‐OxLDL was reported to have atheroprotective association in another interesting study.29 Furthermore, we reported that MDA‐modified LDL‐like antibodies (against phosphorylcholine and against oxidized LDL14) were a significant protection marker for development of atherosclerosis among hypertensives,14 a finding in line with a report from another group.30
Even though it appears that several studies lately favor the notion that anti‐OxLDL (including anti‐MDA LDL) are protection markers, a recent study indicates that anti‐OxLDL (MDA‐modified) was associated with increased risk and was also a marker of LDL‐oxidation.31
One approach is to focus on the smaller parts of modified LDL as the protein apoB‐100 or peptides from apoB‐100, with or without MDA modification. Of note, not only lipids are oxidized during LDL oxidation, but apoB‐100 can also be oxidatively modified. In 1 study, the native apoB‐100‐peptide was a protection marker (which was not the case when MDA‐modified).32 In another study, where the apoB‐100 sequences were studied systematically, MDA‐modified apoB100‐peptides were risk markers for both atherosclerosis and development of CVD with median time from blood sampling to coronary event being as short as 2.8 years.33
Another caveat with antigens in OxLDL, which could play a role in studies where they are risk markers, is that anti‐OxLDL cross‐reacts with anti‐cardiolipin antibodies.34 These antibodies are known to be thrombogenic in autoimmune diseases, especially systemic lupus erythematosus (SLE) and antiphospholipid antibody syndrome,35 but are not associated with atherosclerosis in SLE, only thrombosis.36 Anti‐cardiolipin antibodies are risk markers when present at very high levels, typically above 2 to 3 SD (methods show some variation). This is in contrast to anti‐MDA (and anti‐PC) and in this study, anti‐MDA at very high levels did not show such associations. Instead, anti‐MDA was a strong protection marker at the highest decile. Similar results were obtained in SLE in relation to atherosclerosis and vulnerable plaques.13 Anti‐MDA is thus unlikely to have antiphospholipid‐like properties, so these must reside in some other component in the lipid or protein moiety of OxLDL.
Another explanation to the apparently discrepant data on the role of antibodies to OxLDL‐related compounds is the possibility of immune complex formation, which has been reported as a risk marker in CVD.37 It is interesting to note that ß2GP‐I is also implicated in OxLDL immunity, forming complexes with OxLDL, which could induce T‐cell activation.38
Since data on the role of anti‐OxLDL (including apoB‐100)–related antibodies give discrepant results, we have instead focused on 1 important antigen, the small lipid‐related epitope phosphorylcholine (PC) and chose not to use other components of OxLDL including apoB‐100, as antigen but instead PC associated by other carriers. PC is exposed and recognized by immune‐competent cells both in OxLDL and in infections as parasites and nematodes, among others.2 We reported for the first time that anti‐PC is a protection marker for atherosclerosis development14 and different types of CVD and also in autoimmune diseases such as rheumatoid arthritis and SLE anti‐PC.2, 39
Since MDA is an important small epitope, like PC, and is exposed on OxLDL (but not to the same extent on infectious agents as PC and in oxidative stress and during lipid peroxidation of cell membranes), we chose a strategy where MDA is conjugated with a carrier independent of OxLDL, human albumin, to avoid potential confounders as those discussed. Little is known about the role of antibodies against MDA on other carriers than LDL or LDL‐related proteins/peptides in the development CVD, though 1 recent cross‐sectional study described differential associations depending on the isotype.40
Our findings thus gave comparable data for anti‐MDA as for anti‐PC—low levels associate with high risk, with anti‐MDA also being a protection marker at high levels, with a significantly lower risk.18
These data are also in line with our recent findings, where we reported that IgM anti‐MDA is negatively associated with atherosclerosis in SLE. To identify mechanisms that could explain the protective role of IgM anti‐MDA, we extracted IgM anti‐MDA from total IgM and used FT (containing antibodies not binding to MDA) as a control. We reported that IgM anti‐MDA increase macrophage uptake of apoptotic cells and decrease oxidative stress, which could represent underlying mechanisms, not least in SLE. Both IgM anti‐PC and IgM anti‐MDA were unexpectedly T‐cell dependent.13 The findings presented were recently highlighted in an Editorial.41
When characterizing and comparing the IgM anti‐MDA versus the non‐anti‐MDA FT profiles using a proteomics–de novo sequencing approach, it was apparent that the profiles were different. As expected, the non‐anti‐MDA FT samples contained a more heterogeneous mixture of variable region–derived peptides compared to the IgM anti‐MDA samples that constituted approximately only 20% of the complexity found in the FT. In addition to the overall significantly (P=0.03) lower abundance of identified lambda chains in the anti‐MDA samples, a number of peptides, most prominently 2 peptide pairs assigned to HV CDR3 (P=0.01) and KV CDR2 (P=0.001), were distinctly elevated in anti‐MDA IgMs (Figure 3B). Considering that polyclonal anti‐MDA IgMs are directed to the same antigen, this likely puts restraint on sequence variability; thus, it is not surprising that the anti‐MDA IgM is more homologous and less complex in its content compared to the FT. Generally, sequence homology of antigen‐specific antibodies has been observed in human diseases.42 How consistent the herein presented “anti‐MDA IgM profile” is on the individual subject level still remains to be answered though.
Significance
Our findings indicate that IgM anti‐MDA is a risk marker at lower levels and a protection marker for CVD at the highest levels. Anti‐MDA IgM where OxLDL, LDL, or compounds related to it are not carriers of MDA could also be a novel protection marker in men for development of CVD at higher levels of anti‐MDA. In the future, raising levels of anti‐MDA through immunization could thus be a promising therapeutic possibility.
Sources of Funding
This study was supported by the Swedish Heart Lung Foundation, the Swedish Research Council, the Stockholm County (ALF), the King Gustav V 80th Birthday Fund, Swedish Association against Rheumatism, Vinnova, Torsten Söderberg′s Foundation. The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115565, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007‐2013) and EFPIA companies’ in‐kind contribution. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e004415 doi: 10.1161/JAHA.116.004415)
References
- 1. Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro‐inflammatory (Th1) and macrophage‐stimulating cytokines. Atherosclerosis. 1999;145:33–43. [DOI] [PubMed] [Google Scholar]
- 2. Frostegard J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor‐deficient rabbits with homologous malondialdehyde‐modified LDL reduces atherogenesis. Proc Natl Acad Sci USA. 1995;92:821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, Kaufmann SH, Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992;12:789–799. [DOI] [PubMed] [Google Scholar]
- 5. Fogelman AM, Shechter I, Seager J, Hokom M, Child JS, Edwards PA. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte‐macrophages. Proc Natl Acad Sci USA. 1980;77:2214–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duryee MJ, Klassen LW, Schaffert CS, Tuma DJ, Hunter CD, Garvin RP, Anderson DR, Thiele GM. Malondialdehyde‐acetaldehyde adduct is the dominant epitope after MDA modification of proteins in atherosclerosis. Free Radic Biol Med. 2010;49:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C‐reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. [DOI] [PubMed] [Google Scholar]
- 8. Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, Di Angelantonio E, Gudnason V, Rumley A, Lowe GD, Jorgensen T, Danesh J. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta‐analysis. Eur Heart J. 2014;35:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- 10. Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apoB‐100‐containing lipoproteins: a biomarker predicting cardiovascular disease and cardiovascular events. Biomark Med. 2011;5:673–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zalewski A, Nelson JJ, Hegg L, Macphee C. Lp‐PLA2: a new kid on the block. Clin Chem. 2006;52:1645–1650. [DOI] [PubMed] [Google Scholar]
- 12. Halldin M, Rosell M, de Faire U, Hellenius ML. The metabolic syndrome: prevalence and association to leisure‐time and work‐related physical activity in 60‐year‐old men and women. Nutr Metab Cardiovasc Dis. 2007;17:349–357. [DOI] [PubMed] [Google Scholar]
- 13. Rahman M, Sing S, Golabkesh Z, Fiskesund R, Gustafsson T, Jogestrand T, Frostegard AG, Hafstrom I, Liu A, Frostegard J. IgM antibodies against malondialdehyde and phosphorylcholine are together strong protection markers for atherosclerosis in systemic lupus erythematosus: regulation and underlying mechanisms. Clin Immunol. 2016;166–167:27–37. [DOI] [PubMed] [Google Scholar]
- 14. Su J, Georgiades A, Wu R, Thulin T, de Faire U, Frostegard J. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis. 2006;188:160–166. [DOI] [PubMed] [Google Scholar]
- 15. Barnidge DR, Lundstrom SL, Zhang B, Dasari S, Murray DL, Zubarev RA. Subset of kappa and lambda germline sequences result in light chains with a higher molecular mass phenotype. J Proteome Res. 2015;14:5283–5290. [DOI] [PubMed] [Google Scholar]
- 16. Zhang B, Pirmoradian M, Chernobrovkin A, Zubarev RA. DeMix workflow for efficient identification of cofragmented peptides in high resolution data‐dependent tandem mass spectrometry. Mol Cell Proteomics. 2014;13:3211–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang B, Kall L, Zubarev RA. DeMix‐Q: quantification‐centered data processing workflow. Mol Cell Proteomics. 2016;15:1467–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Faire U, Su J, Hua X, Frostegard A, Halldin M, Hellenius ML, Wikstrom M, Dahlbom I, Gronlund H, Frostegard J. Low levels of IgM antibodies to phosphorylcholine predict cardiovascular disease in 60‐year old men: effects on uptake of oxidized LDL in macrophages as a potential mechanism. J Autoimmun. 2010;34:73–79. [DOI] [PubMed] [Google Scholar]
- 19. Salonen JT, Yla‐Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum JL. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–887. [DOI] [PubMed] [Google Scholar]
- 20. Bergmark C, Wu R, de Faire U, Lefvert AK, Swedenborg J. Patients with early‐onset peripheral vascular disease have increased levels of autoantibodies against oxidized LDL. Arterioscler Thromb Vasc Biol. 1995;15:441–445. [DOI] [PubMed] [Google Scholar]
- 21. Puurunen M, Manttari M, Manninen V, Tenkanen L, Alfthan G, Ehnholm C, Vaarala O, Aho K, Palosuo T. Antibody against oxidized low‐density lipoprotein predicting myocardial infarction. Arch Intern Med. 1994;154:2605–2609. [PubMed] [Google Scholar]
- 22. Wu R, Nityanand S, Berglund L, Lithell H, Holm G, Lefvert AK. Antibodies against cardiolipin and oxidatively modified LDL in 50‐year‐old men predict myocardial infarction. Arterioscler Thromb Vasc Biol. 1997;17:3159–3163. [DOI] [PubMed] [Google Scholar]
- 23. Lehtimaki T, Lehtinen S, Solakivi T, Nikkila M, Jaakkola O, Jokela H, Yla‐Herttuala S, Luoma JS, Koivula T, Nikkari T. Autoantibodies against oxidized low density lipoprotein in patients with angiographically verified coronary artery disease. Arterioscler Thromb Vasc Biol. 1999;19:23–27. [DOI] [PubMed] [Google Scholar]
- 24. Palinski W, Tangirala RK, Miller E, Young SG, Witztum JL. Increased autoantibody titers against epitopes of oxidized LDL in LDL receptor‐deficient mice with increased atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1569–1576. [DOI] [PubMed] [Google Scholar]
- 25. van de Vijver LP, Steyger R, van Poppel G, Boer JM, Kruijssen DA, Seidell JC, Princen HM. Autoantibodies against MDA‐LDL in subjects with severe and minor atherosclerosis and healthy population controls. Atherosclerosis. 1996;122:245–253. [DOI] [PubMed] [Google Scholar]
- 26. Virella G, Virella I, Leman RB, Pryor MB, Lopes‐Virella MF. Anti‐oxidized low‐density lipoprotein antibodies in patients with coronary heart disease and normal healthy volunteers. Int J Clin Lab Res. 1993;23:95–101. [DOI] [PubMed] [Google Scholar]
- 27. Uusitupa MI, Niskanen L, Luoma J, Vilja P, Mercuri M, Rauramaa R, Yla‐Herttuala S. Autoantibodies against oxidized LDL do not predict atherosclerotic vascular disease in non‐insulin‐dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:1236–1242. [DOI] [PubMed] [Google Scholar]
- 28. Wu R, de Faire U, Lemne C, Witztum JL, Frostegard J. Autoantibodies to OxLDL are decreased in individuals with borderline hypertension. Hypertension. 1999;33:53–59. [DOI] [PubMed] [Google Scholar]
- 29. Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin M type of autoantibodies to oxidized low‐density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. [DOI] [PubMed] [Google Scholar]
- 30. Rontu R, Metso S, Jaakkola O, Nikkila M, Jokela H, Lehtimaki T. Antibody titer against malondialdehyde‐modified LDL compares with HDL cholesterol concentration in identifying angiographically verified coronary artery disease. Comparison of tests by ROC analysis. Clin Chem Lab Med. 2005;43:357–360. [DOI] [PubMed] [Google Scholar]
- 31. Maiolino G, Pedon L, Cesari M, Frigo AC, Barisa M, Rossitto G, Seccia TM, Zanchetta M, Rossi GP. Antibodies to malondialdehyde oxidized low‐density lipoproteins predict long term cardiovascular mortality in high risk patients. Int J Cardiol. 2013;168:484–489. [DOI] [PubMed] [Google Scholar]
- 32. Sjogren P, Fredrikson GN, Samnegard A, Ericsson CG, Ohrvik J, Fisher RM, Nilsson J, Hamsten A. High plasma concentrations of autoantibodies against native peptide 210 of apoB‐100 are related to less coronary atherosclerosis and lower risk of myocardial infarction. Eur Heart J. 2008;29:2218–2226. [DOI] [PubMed] [Google Scholar]
- 33. Fredrikson GN, Hedblad B, Berglund G, Alm R, Ares M, Cercek B, Chyu KY, Shah PK, Nilsson J. Identification of immune responses against aldehyde‐modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:872–878. [DOI] [PubMed] [Google Scholar]
- 34. Vaarala O, Alfthan G, Jauhiainen M, Leirisalo‐Repo M, Aho K, Palosuo T. Crossreaction between antibodies to oxidised low‐density lipoprotein and to cardiolipin in systemic lupus erythematosus. Lancet. 1993;341:923–925. [DOI] [PubMed] [Google Scholar]
- 35. Hughes GR, Harris NN, Gharavi AE. The anticardiolipin syndrome. J Rheumatol. 1986;13:486–489. [PubMed] [Google Scholar]
- 36. Frostegard AG, Su J, Hua X, Vikstrom M, de Faire U, Frostegard J. Antibodies against native and oxidized cardiolipin and phosphatidylserine and phosphorylcholine in atherosclerosis development. PLoS One. 2014;9:e111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lopes‐Virella MF, Hunt KJ, Baker NL, Virella G, Moritz T; Investigators V . The levels of MDA‐LDL in circulating immune complexes predict myocardial infarction in the VADT study. Atherosclerosis. 2012;224:526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsuura E, Kobayashi K, Matsunami Y, Lopez LR. The immunology of atherothrombosis in the antiphospholipid syndrome: antigen presentation and lipid intracellular accumulation. Autoimmun Rev. 2009;8:500–505. [DOI] [PubMed] [Google Scholar]
- 39. Frostegard J. Low level natural antibodies against phosphorylcholine: a novel risk marker and potential mechanism in atherosclerosis and cardiovascular disease. Clin Immunol. 2010;134:47–54. [DOI] [PubMed] [Google Scholar]
- 40. Anderson DR, Duryee MJ, Shurmur SW, Um JY, Bussey WD, Hunter CD, Garvin RP, Sayles HR, Mikuls TR, Klassen LW, Thiele GM. Unique antibody responses to malondialdehyde‐acetaldehyde (MAA)‐protein adducts predict coronary artery disease. PLoS One. 2014;9:e107440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McMahon M, Skaggs B. Autoimmunity: do IgM antibodies protect against atherosclerosis in SLE? Nat Rev Rheumatol. 2016;12:442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]


