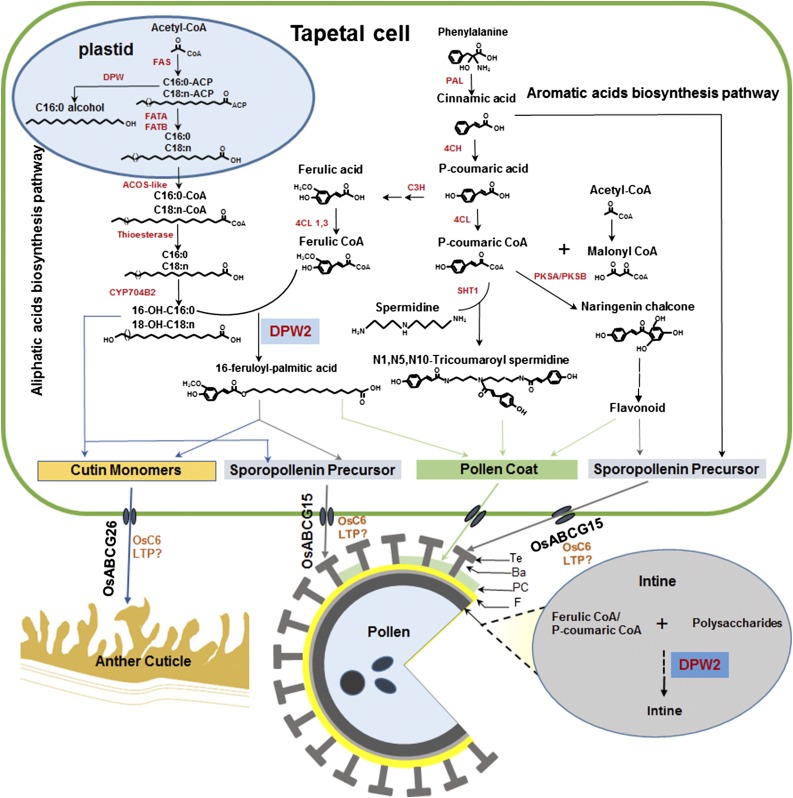
Figure 11.
The proposed roles of DPW2 in anther cuticle and pollen wall development. In tapetal cells, fatty acids are synthesized de novo in plastids from acetyl-CoA to a maximum of C16 and C18 in length, followed by esterification to long-chain acyl carrier proteins (ACPs). Then the product is either reduced by DPW to fatty alcohols or further hydrolyzed by Acyl-ACP thioesterases (FATA and FATB) to release the free fatty acids and exported from the plastid to the cytoplasm, where they are modified via thioesterase and CYP450 hydroxylase. The resulting hydroxylated fatty acids are either used directly as the precursors for anther cutin and pollen sporopollenin or cross linked with aromatic acids generated from phenylpropanoid pathway by DPW2 to produce various precursors to be transported by ABCG (such as OsABCG15 and OsABCG26) or lipid transport proteins (LTP, such as OsC6). DPW2 may also cross link aromatic acids with polysaccharides for pollen intine biosynthesis. 4CL, 4-Coumarate:CoA ligase; ACOS-like, acyl-CoA synthase-like; C3H, coumaric acid 3-hydroxylase; C4H, cinnamate 4-hydroxylase; CYP704B2, cytochrome P450 of 704B2; DPW, defective pollen wall; FATA/FATB, fatty acyl thioesterase A (B); PAL, Phe ammonia-lyase; PKSA/PKSB, polyketide synthase a/polyketide synthase b; SHT1, spermidine hydroxycinnamoyl transferase 1; Ba, bacula; F, foot layer; PC, pollen coat; Te, tectum.