Abstract
Virus-induced flowering combines fundamental research in reproductive biology with efficient tools for manipulating gene expression in nonmodel systems to accelerate discovery and breeding.
For many crops, the benefits of traditional breeding practices are difficult to achieve because the crops have long juvenile periods that necessitate long breeding cycles, desired genotypes for a cross do not have synchronized flowering because of photoperiod or vernalization requirements, or preferred lines are propagated asexually and have limited flowering potential. In light of these limitations, and being faced with the desire to accelerate breeding as a means of contending with climate change, dwindling water resources, and invasive biotic stressors, we explore the use of virus-induced flowering (VIF) as a method to accelerate the transition to reproductive growth and thus facilitate research and breeding.
REGULATED FLOWERING IMPEDES BREEDING
Plant breeding requires the production of fertile flowers to produce the necessary gametes. However, obtaining such flowers in a synchronized manner and in a practical period of time can be challenging. The transition to flowering requires the coordination of developmental and environmental signals (Amasino, 2010). Many plants experience an extended juvenile phase before being capable of transitioning to reproductive growth, and this can severely delay the development of new lineages with preferred traits. Flowering after the juvenile phase in fruit and nut trees are classic examples, with almond (Prunus dulcis), cherry (Prunus avium), and peach (Prunus persica) requiring 3 to 5 years, citrus (Citrus spp.) trees requiring 5 to 10 years, and apple (Malus × domestica) and pear (Pyrus communis) requiring 6 to 12 years; developing new cultivars can span decades (van Nocker and Gardiner, 2014). Breeding programs for fruit and nut trees with superior fruit traits, disease resistance, and postharvest physiology are adopting advanced technologies such as marker-assisted breeding and genome-wide association mapping to make these long breeding cycles as efficient as possible (Hardner et al., 2016; Iwata et al., 2016). Fast-growing hardwood trees like poplar (Populus spp.) and eucalypts (Eucalyptus spp.) are valuable for pulp, energy, and timber, but developing lineages optimized for these applications are similarly constrained by juvenile periods. While some eucalypt genotypes can flower in 2 to 3 years, other commercially important species, such as Eucalyptus globulus, Eucalyptus dunnii, and Eucalyptus nitens, often take 10 or more years to flower (Eldridge et al., 1993; Missiaggia et al., 2005; Sein and Mitlohner, 2011). Rapid flowering would accelerate conventional breeding and help realize benefits from genomic selection methods.
Vernalization, the process of prolonged chilling to stimulate flowering, also can constrain when and where flowering occurs. Winter annuals, typified by winter cereals, respond to chilling early in their life cycle. They are planted in the fall, go dormant over winter while vegetative, and flower in the spring. The Green Revolution promoted the breeding of spring cereals that matured without a vernalization requirement (Chrispeels and Sadava, 2003). Biennials must reach a minimum size before becoming chilling sensitive and, thus, grow vegetatively for a full season and flower the following summer. Chilling requirements for flowering are common among temperate fruit crops and deciduous trees, and the number of chilling hours required can restrict the production range (Srinivasan et al., 2012).
Photoperiodism also poses a challenge for breeders. Many modern crops are day neutral but are derived from photoperiodic ancestors. Without exception, the selection of day-neutral flowering introduced a genetic bottleneck. For example, cultivated cotton (Gossypium hirsutum) is a day-neutral plant and has one of the most narrow genetic bases of all commodity crops (Iqbal et al., 2001; Paterson et al., 2004). Landraces and wild accessions are important reserves for desirable traits such as tolerance to diseases, pests, drought, and salt, but these are short-day photoperiodic plants. There is interest in crossing this diversity into cultivated cotton for crop improvement, but differences in the onset of flowering complicate breeding and increase costs (Iqbal et al., 2001; Paterson et al., 2004; Robinson, 2007; Saha et al., 2008). The situation with sorghum (Sorghum bicolor) is similar, and in both crops, significant conversion programs are underway to introduce day-neutral flowering to wild accessions to make this diversity more available to breeders (Kimber et al., 2013; Klein et al., 2013)
Finally, some crops are propagated primarily vegetatively and/or elite lines have low fertility, greatly impeding the production of improved varieties. Among those in which the harvested organs are not seed or fruit, flowers are competing sinks. Consequently, varieties that do not flower, or that have weak flowering, are the ones that are often cultivated. Sugarcane (Saccharum spp.), miscanthus (Miscanthus spp.) biofuel grasses, and cassava (Manihot esculenta) are examples. Cassava feeds more than half a billion people, but clonal propagation renders this important dietary staple vulnerable to disease epidemics and abiotic stresses (Ceballos et al., 2004; Sayre et al., 2011; Bredeson et al., 2016). Similar concerns pertain to many other low-diversity crops.
HOW CAN BREEDING BE ACCELERATED?
Traditional breeding among varieties with specific developmental or environmental stimuli relies upon moving the breeding program to locations where those stimuli occur naturally on a seasonal basis or mimicking those stimuli in horticultural manipulations, such as modified photoperiodic environments; both options can be costly. Other breeding tools that induce early flowering include grafting nonflowering accessions onto flowering stock plants, the use of naturally early-flowering cultivars (Bolotin, 1975; Missiaggia et al., 2005), and hormone applications (Griffin et al., 1993; Williams et al., 2003). While important, these approaches take time and considerable research to optimize, and often they are not effective for cultivars of interest.
Increasingly, the transition to reproductive growth is being accelerated through the ectopic expression of FLOWERING LOCUS T (FT), which encodes the long-distance flowering signal florigen (Zhang et al., 2010; Srinivasan et al., 2012; Wenzel et al., 2013). The FT signal is well characterized in Arabidopsis (Arabidopsis thaliana; AtFT), and extensive research across diverse plant species shows that FT and FT-like genes coordinate the environmental and endogenous signals governing the transition to reproductive growth (Lifschitz et al., 2006, 2014; Zeevaart, 2008). TERMINAL FLOWER1 (TFL1) is in the same gene family as FT, but TFL1 (and homologs) acts as a competitive inhibitor of FT (Hanano and Goto, 2011; Ho and Weigel, 2014; Lifschitz et al., 2014). Consequently, either FT gain of function or TFL1 loss of function results in more determinate growth and faster transition to reproductive growth (Flachowsky et al., 2012; Freiman et al., 2012). Other genes in the flowering pathway are used similarly as breeding tools to accelerate flowering; for example, apple transformed with a flowering-associated MADS box gene from silver birch (Betula spp.; BdMADS4) demonstrates precocious flowering and permits one breeding cycle per year (Flachowsky et al., 2011; Weigl et al., 2015).
Overexpressing FT generally accelerates the transition to reproductive growth, uncouples flowering from photoperiod and vernalization requirements, and in perennial trees acts downstream of, and thereby bypasses, the juvenile phase (Endo et al., 2005; Böhlenius et al., 2006; Hsu et al., 2006). Overexpressing FT in trees such as eucalypts promotes flowering and yields fertile flowers with viable pollen and seeds (Fig. 1; Klocko et al., 2016), but as with most transgenes, the threshold level of expression can be variable among events. Apple transformed with MdFT1, citrus transformed with CiFT, and plum (Prunus domestica) transformed with CiFT and PtFT yielded fertile flowers, pollen, and seeds, respectively (Endo et al., 2005; Kotoda et al., 2010; Tränkner et al., 2010; Srinivasan et al., 2012). Constitutive FT expression in these transgenic lines resulted in very early flowering. More controlled flowering was achieved in poplar using a heat-inducible FT gene (Wenzel et al., 2013); however, obtaining viable pollen and seed was challenging (Zhang et al., 2010; Hoenicka et al., 2014) and required specialized growth conditions (Hoenicka et al., 2016).
Figure 1.
Overexpressing AtFT in transgenic eucalypts overcomes the juvenile phase and promotes the early transition to reproductive growth. A, A wild-type eucalypt shows only vegetative growth during the juvenile phase of development. B, Ectopic expression of the AtFT transgene from the 409S promoter results in precocious flowering. Floral buds are evident at the apices of branches (arrows). The inset shows an induced eucalypt flower at anthesis.
The use of transgenic lines with precocious flowering is colloquially called rapid cycling or FasTrack breeding and usually is coupled with marker-assisted selection (MAS) for desired traits (van Nocker and Gardiner, 2014; Callahan et al., 2015; Weigl et al., 2015). The principle is that a commercially important lineage is converted to constant flowering by introducing an FT-like transgene. This line is crossed with another parent harboring desirable traits, such as disease resistance. Since the transgene for flowering is both dominant and hemizygous, progeny plants segregating for early flowering and for the introgressed trait of interest are selected, aided by MAS. Without having to wait through a juvenile period, these offspring then can be backcrossed to the desired recurrent parent on more-or-less annual cycles. In addition, adult traits such as fruit quality may be tested during the first year of growth, rather than waiting through the juvenile period, as is required in conventional breeding. Once the desired crosses are complete, the hemizygous FT transgene is segregated away, and only then does the breeder have to wait through a juvenile period to test variety potential. Because the transgene is ultimately removed, the final genotype is not transgenic, and this may facilitate acceptance by regulatory agencies and the general public (van Nocker and Gardiner, 2014; see below).
However, accelerated flowering through transgene overexpression can be problematic for a number of reasons. Standard transformation strategies are time consuming and require skilled labor; they are inefficient in many species and usually are applicable in a limited number of genetic backgrounds; and many important crop varieties and species remain recalcitrant. The restriction of transformation to a small number of genetic backgrounds can have large impacts on breeding progress (Wallace et al., 2009; Altpeter et al., 2016). In addition, although somaclonal variation is an explicit breeding method in many species, it can introduce undesired genetic and epigenetic changes (Kaeppler et al., 2000). Somaclonal variation is commonly observed during conventional as well as transgenic in vitro regeneration methods. In addition, graft transmission of FT-induced transgenic flowering is difficult; despite abundant flowering from transgenic poplar rootstocks, no flowering was observed when wild-type scions were grafted to FT-overexpressing rootstocks (Zhang et al., 2010). A method that is less species and genotype dependent, is readily graft transmissible, and does not require slow and complex in vitro methods would be highly desirable.
VIRUS-INDUCED FLOWERING: EFFICIENT TRANSIENT INDUCTION OF FLOWERING
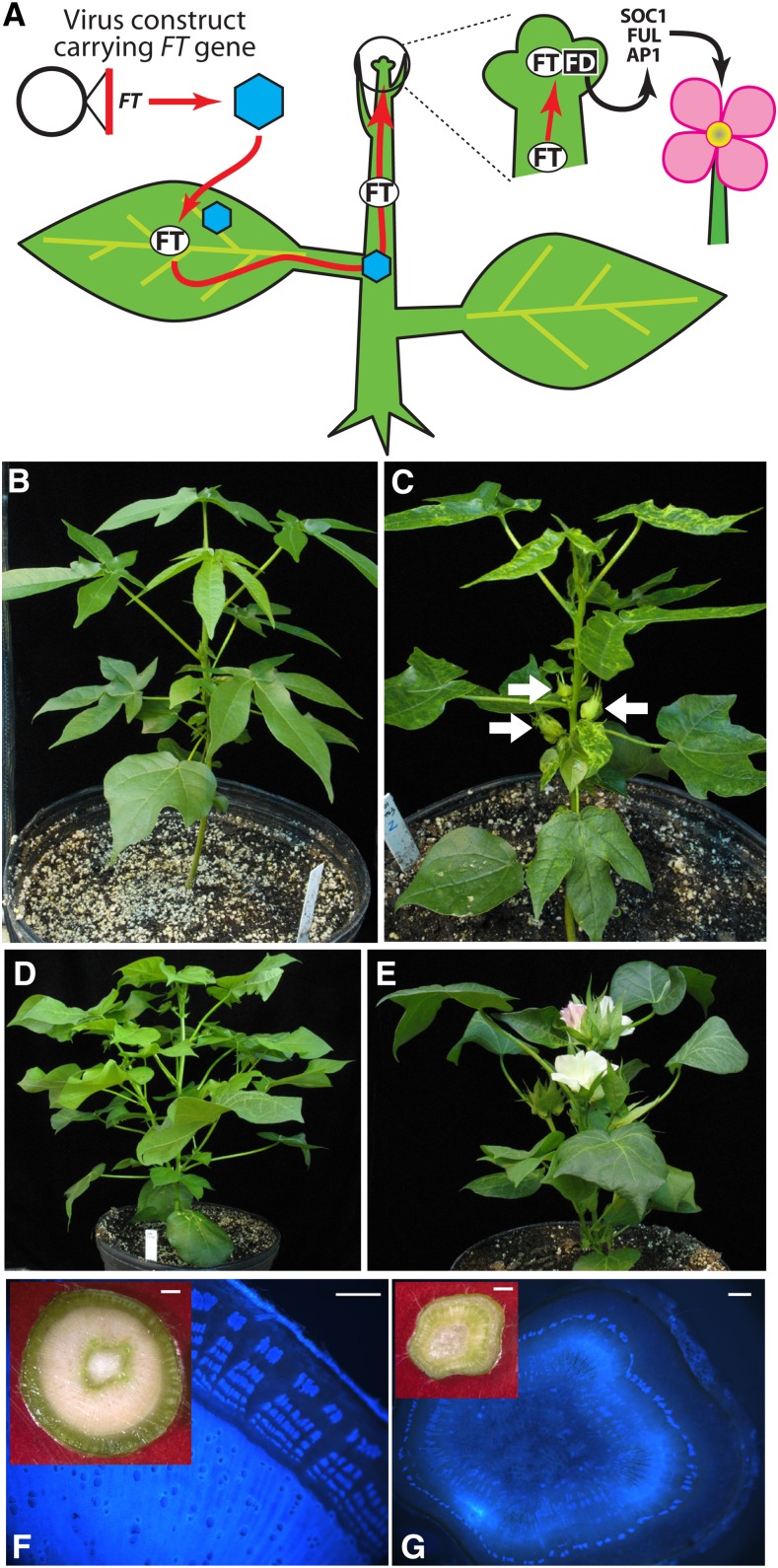
As an alternative to stable transformation, viral vectors have been used to deliver FT orthologs to different crop plants to induce determinate growth patterns and precocious flowering (Fig. 2A). This approach makes sense: the FT gene product, florigen, is phloem mobile and naturally finds its way into apices to influence meristem identity, and viruses use the phloem as a pathway to establish systemic infections. Therefore, in principle, coupling an FT ortholog with a virus-based vector that can amplify the inserted sequence and move it systemically will promote flowering. As an allusion to the popular technique of virus-induced gene silencing (VIGS), the use of a virus to deliver sequences that promote flowering was termed VIF (McGarry and Ayre, 2012a). Because virus infection progresses through whole plants, techniques for transformation in sterile tissue culture do not need to be developed, and there is no risk of somaclonal variation. Table I summarizes potential advantages of VIF over transgenic approaches to induce flowering.
Figure 2.
Virus-induced flowering (VIF). A, Cartoon depicting how VIF works (adapted from McGarry and Ayre, 2012a). B, An uninoculated photoperiodic cotton plant (accession TX 701) grown under noninductive long days (16 h of light/8 h of dark) demonstrates exclusively vegetative growth. C, When the cotton FT ortholog, GhSFT, is delivered from dCLCrV, flowering is uncoupled from photoperiod and the transition to reproductive growth is accelerated in accession TX 701. Arrows point to induced floral buds. D, An uninoculated day-neutral cotton plant (accession Delta Pine 61) produces robust vegetative growth along with many floral buds. E, Silencing the cotton TFL1 homolog, GhSP, from TRV terminates growth by node 5 with the formation of a terminal flower, and all axillary meristems are converted to flowers. In both C and E, the induced flowers were fertile and set fruit (data not shown). F, Cross section of the main stem from a mature, uninoculated day-neutral cotton (accession Delta Pine 61). The inset shows a cross section visualized with reflected light (bar = 1 mm). The autofluorescence of lignin polymers is visualized with UV epifluorescence. Secondary xylem and stacks of phloem fibers are clearly organized (adapted from McGarry et al., 2016). Bar = 500 µm. G, Cross section of the main stem from a GhSP-silenced accession Delta Pine 61 plant of the same age as in F. A reflected light image is shown in the inset (bar = 1 mm). UV fluorescence reveals scant and scattered secondary xylem, short stacks of phloem fibers, and expanded cortex. Bar = 500 µm. All plants shown were grown under long-day conditions (16 h of light/8 h of dark).
Table I. Advantages and disadvantages of VIF compared with approaches involving stable transgenic lines.
| Pros | Cons |
|---|---|
| Eliminates time and labor in transforming recalcitrant plants and regenerating transformants | Transmission of virus to the next generation may be variable, and as in transgenics, the absence of recombinant materials must be verified |
| Curtails the risk of somaclonal variation arising through tissue culture | Each virus has its own host range that can limit its utility across species |
| Most plant viruses do not integrate into the host genome and are not transmitted efficiently to seeds | FT expression is controlled by viral promoters, making inducible or tissue-specific expression difficult to attain |
| FT and virus are phloem mobile, thus amplifying the flowering signal and improving the rate of floral induction | Regulations for VIF plants and progeny derived from VIF are largely untested outside of laboratories and research greenhouses |
| Cloned viruses used for VIGS can silence TFL1 homologs to induce flowering | Public acceptance of products obtained via VIF and verified to be free of recombinant sequences is unknown |
The first demonstration of VIF used Zucchini yellow mosaic virus to deliver FT to cucurbits, stimulating flowering in short-day melon (Cucurbita moschata) under noninductive long days (Lin et al., 2007). This early work demonstrated that FT encoded florigenic properties and laid the foundation for VIF as a breeding tool. Work in cotton demonstrated that ectopic expression of AtFT from a disarmed Cotton leaf crumple virus (dCLCrV) vector uncoupled flowering from photoperiod, accelerated the transition to reproductive growth, and enhanced determinate plant architecture (McGarry and Ayre, 2012b). Ectopic expression of AtFT in soybean (Glycine max) with both determinate or indeterminate stem habits, when delivered by Apple latent spherical virus (ALSV), terminated vegetative growth without an inductive short-day treatment (Yamagishi and Yoshikawa, 2011). ALSV:AtFT also was introduced to apple seedlings, and these seedlings flowered within 1 month, thus overcoming apple’s extended juvenile phase (Yamagishi et al., 2011). The induced apple and cotton floral buds were used as pollen donors to successfully introduce exotic germplasm into inbred domesticated varieties. In both cases, the F1 progeny displayed phenotypes intermediate to both parents, demonstrating the success of the cross. Importantly, neither dCLCrV nor ALSV was detected in the F1 generation (Yamagishi et al., 2011; McGarry and Ayre, 2012b).
In a citrus breeding program, Citrus leaf blotch virus (CLBV) was used to deliver AtFT and CiFT (the FT ortholog from Valencia orange [Citrus sinesis]), and precocious flowering occurred within 4 to 6 months instead of the typical more than 6 years. Other than dramatically reducing the juvenile period, flowering and fruiting were not affected (Velázquez et al., 2016). In this breeding program, tetraploid hybrids are created by sexual hybridization and screened by MAS for desirable traits. Selected hybrids are then inoculated with the CLBV-based vector carrying FT to induce precocious flowering and are crossed with diploid parents to create seedless triploid progeny. These authors report that more than 1,600 crosses have been made with this method (Velázquez et al., 2016).
In addition to FT gain of function, determinate growth habits also have been induced through VIGS of the FT antagonist TFL1. This is important for virus-mediated breeding applications, since a larger diversity of viruses have been adapted for VIGS than for virus-mediated gain of function and may be more effective. VIF using AtFT or cotton SINGLE FLOWER TRUSS (GhSFT; SFT is the FT ortholog in tomato [Solanum lycopersicum]) gain of function rapidly induced the onset of flowering, and growth was more determinate than in naturally reproductive plants (Fig. 2, B and C; McGarry and Ayre, 2012b; McGarry et al., 2016). However, VIGS of SELF-PRUNING (GhSP; a tomato homolog of TFL1) using Tobacco rattle virus (TRV) more severely altered growth habit. After producing five nodes, the monopodial main stem, which normally remains vegetative throughout the life cycle, terminated with an apical flower, and axillary buds released from apical dominance immediately transitioned to reproductive growth, including those at the cotyledonary node (Fig. 2, D and E; McGarry et al., 2016). The resulting phenotype was extreme, producing a cotton plant about 20 cm tall with no branching but with six flowers. Plants with this diminutive phenotype would have little value for field production, but as a breeding tool, the approach could accelerate the production of pollen, ovules, embryos, and seeds from elite breeding lines. In apple, ALSV was used to simultaneously deliver AtFT function and silence MdTFL1 (Yamagishi et al., 2014). Infected plants similarly showed precocious determinate growth and reduced breeding cycles to less than a single year.
Virus-based technologies also inform about reproductive and general biological processes in nonmodel species that are difficult to study through other standard techniques in genetics or molecular biology. In cotton, virus-based gain and loss of function of GhSFT and GhSP showed how these genes contribute to overall plant architecture. Specifically, these studies supported architectural models developed in tomato that argue that the ratio of SFT-like activities and SP-like activities controls the pattern of determinate and indeterminate growth at all meristems in the shoot (Shalit et al., 2009). Altering the GhSFT/GhSP balance by delivering GhSFT made plants more determinate by overcoming photoperiod requirements and reduced vegetative growth, causing sympodial fruiting branches to terminate in floral clusters. Altering the GhSFT/GhSP balance by silencing GhSP made plants extremely determinate, as described above, but these effects extended to all shoot meristems. Lobed leaves were reduced to simple lanceolate leaves, indicating that leaf marginal meristems became more determinate, and xylem and phloem growth was curtailed dramatically, indicating that the cambial meristems also were more determinate (Fig. 2, F and G; McGarry et al., 2016). These results imply a role of the SFT/SP (or FT/TFL1) balance in regulating woody versus herbaceous stem growth. Virus-based gene manipulations also showed that AtFT and GhSFT functions are not identical: plants infected with dCLCrV:AtFT produced a significant proportion of abnormal, sterile flowers, while plants infected with dCLCrV:GhSFT produced only normal, fertile flowers (McGarry and Ayre, 2012b; McGarry et al., 2013, 2016). Why the two genes differentially impact floral development is not currently known, but the efficient delivery of different genes has exposed potential differences in function. Abnormal flowers also were noted in poplar with ectopic FT expression (Zhang et al., 2010).
VIF: A POWERFUL TOOL IN A SPECTRUM OF RECALCITRANT PLANTS?
VIF could be a useful tool for accelerating discovery and breeding in a broad spectrum of plants in which natural reproduction constrains breeding rate. For example, cassava is an important food and emerging biofuel crop in tropical areas. Cassava is predominantly clonally propagated, although breeding programs for improved nutritional and disease resistance are under way (Ceballos et al., 2004; Sayre et al., 2011). Cassava mosaic virus is a geminivirus developed previously for VIGS (Fofana et al., 2004) and is a promising tool for VIF. Miscanthus × giganteus, a triploid sterile hybrid, is an appealing perennial bioenergy crop with high biomass production and low environmental impact. However, only a few clones exist, and biomass potential varies in different climates, emphasizing the need for region-specific varieties (Sacks et al., 2013; Arnoult and Brancourt-Hulmel, 2015). Sugarcane is valued for food, ethanol, and biomass production, and there is a need for new varieties with high sugar and biomass yield. Modern cultivars are highly polyploid, and this genetic complexity challenges standard and molecular breeding programs. The transition to flowering in sugarcane also has genotype-specific requirements for plant age and size, photoperiod, temperature, and humidity, such that the range for breeding under natural conditions is limited. In other environments where vegetative growth is robust but inductive conditions for flowering do not occur, breeding is accomplished in large greenhouses that can mimic the necessary conditions. Introducing a new cultivar takes up to 12 years (Paterson et al., 2013; Racedo et al., 2016). Sorghum is naturally a short-day plant and is cultivated as grain, forage, sweet, and high biomass varieties (Kimber et al., 2013; Morris et al., 2013). Grain sorghums were selected for early flowering in temperate regions (Murphy et al., 2011). Forage and sweet lines flower later for more vegetative growth, and biomass lines retain full short-day photoperiodism to maximize yields for biofuels during the long-day growing season (Wolabu et al., 2016). VIF has yet to be demonstrated in any of these grasses, but each would benefit from tools to facilitate breeding. In addition, hybrids demonstrating heterosis contribute dramatically to agricultural productivity, and mechanisms to synchronize flowering across diverse parental lines may greatly facility hybrid production. Viruses adapted to alter gene expression in monocots lag behind the number available for dicot plants. Vectors harboring Brome mosaic virus and Barley stripe mosaic virus are used for VIGS (Ding et al., 2006; Scofield and Nelson, 2009; Yuan et al., 2011), and Foxtail mosaic virus was shown recently to be effective for VIGS in C4 grasses, including foxtail millet (Setaria italica), maize (Zea mays), and sorghum (Liu et al., 2016; Mei et al., 2016). The organization of these viruses may be more suited to VIF by silencing TFL1 homologs.
REAL-WORLD CONSIDERATIONS: CAN VIF DEVELOP AS A WIDELY USED TOOL?
The development of VIF into a widely used tool requires additional research. The greatest limitation to broad adoption is finding a virus suitable for the crop of interest. Virus-host interactions are tightly linked through evolution, and although some viruses have a broad host range, the effectiveness of a virus for a crop of interest needs to be tested. This situation is complicated further by genotype-specific susceptibility to infection. Fortunately, a number of viruses have been developed for VIGS by the research community, and these can be used directly to silence TFL1 homologs or adapted to deliver FT activity (Robertson, 2004). Beyond those mentioned previously, a survey of these viruses is beyond the scope of this article, but the positive-strand RNA virus TRV is effective in a diversity of dicots (Liu et al., 2002; Hileman et al., 2005) and several single-stranded DNA geminiviruses are developed for VIGS in specific lineages (Carrillo-Tripp et al., 2006). As mentioned previously, there is limited development of virus-based vectors for monocots.
Generally, most plant viruses do not integrate into the host genome and do not pass efficiently through the germline, such that seeds are virus free (Johansen et al., 1994). These characteristics of virus-based approaches provide technical and perhaps regulatory advantages over transgenic approaches, but the absence of viral sequences in progeny needs to be thoroughly tested and confirmed, as some viruses currently used for VIGS and VIF are transmitted at low levels to the next generation. TRV that is used widely for VIGS, and CLBV used for VIF in citrus, were both detected by PCR in less than one in 100 seeds analyzed in Arabidopsis and citrus, respectively (Senthil-Kumar and Mysore, 2011; Velázquez et al., 2016). ALSV and dCLCrV were not detected in progeny after VIF (Yamagishi et al., 2011; McGarry and Ayre, 2012b; Yamagishi et al., 2014); however, consistency of nontransmission requires further analysis, preferably with additional genotypes, environments, and larger numbers of progeny.
In rapid-cycling FasTrack fruit-breeding programs relying upon FT transgenic plants in crosses, the transgene is eliminated in the final population through segregation. In the United States, the U.S. Department of Agriculture Animal and Plant Health Inspection Service Biotechnology Regulatory Services has set a precedent that null segregants are not regulated articles (USDA, 2011, 2014), but the population needs to be proven as null segregants by phenotypic and molecular analysis. It is reasonable that similar analysis after VIF would render progeny of the VIF approach as nonregulated articles. It is noteworthy that, with a transgenic approach, 50% of the cross progeny from a single-insertion event will carry the FT transgene, whereas approximately 1% of progeny in a VIF approach may have seed transmission of the virus. Although the Animal and Plant Health Inspection Service may not regulate organisms demonstrated to be free of recombinant sequences, they may still be regulated by other agencies in the United States and in other countries. Regulatory procedures are vastly different from country to country (Lusser and Davies, 2013).
The National Institutes of Health Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH, 2016) specifies the practices for constructing and handling of “(i) recombinant nucleic acid molecules, (ii) synthetic nucleic acid molecules, including those that are chemically or otherwise modified but can base pair with naturally occurring nucleic acid molecules, and (iii) cells, organisms, and viruses.” It also describes containment requirements for vectors derived from plant viruses. If less than two-thirds of the viral genome is used in the recombinant vector and the vector does not lead to productive infection, then the viral system would generally require Biosafety Level 1-Plant, typically achieved in any locked greenhouse with window screens recommended to prevent the movement of mammals, birds, and arthropods. Using a larger portion of the viral genome requires more stringent containment, such as Biosafety Level 2-Plant, which is readily met with proven insect-proof screens in greenhouses and by rendering biological material inactive after an experiment. Other important considerations with virus-based constructs include the mode of transmission (e.g. mechanical or via an insect vector), the proximity to natural or agricultural stands that harbor potential host plants, symptoms produced by infection, and the natural distribution of the native virus (e.g. whether the virus is considered a nonexotic or an exotic and whether it has recognized potential for serious detrimental impact on managed or natural ecosystems). Because VIF is intended to ultimately facilitate breeding, the use of any virus backbone requiring containment beyond Biosafety Level 2-Plant is likely impractical to implement.
If VIF were to be used as a commercial breeding method, it is likely to be used in an incompletely contained environment, such as a poorly sealed greenhouse, a lath house, or a field site. In those cases, it would be regulated primarily by the U.S. Department of Agriculture as a recombinant plant pest under part 340 of the Code of Federal Regulations, which covers “introduction of organisms and products altered or produced through genetic engineering which are plant pests or which there is reason to believe are plant pests” (eCFR, 2016a). Unless the virus was proven to be disarmed and incapable of inciting disease or recombining with native viruses, it may remain a regulated article permanently, which may not present a problem for a breeding program. However, the pollen and seeds from VIF-treated plants would be regulated until definitively proven that there is a complete absence of transmission, as discussed above.
As a recombinant microbial, the Environmental Protection Agency also may regulate field releases through the Biotechnology Notification Program in the United States (eCFR, 2016b). The Environmental Protection Agency also may consider a stature- and flowering-modified plant as a growth-regulated plant that falls under the definition of a regulated transgenic plant, although, to our knowledge, to date it has not chosen to regulate growth-modified plants (Strauss and Sax, 2016). Furthermore, unless the virally delivered target gene is incorporated into the plant genome, it would not qualify as a plant-incorporated protectant but would remain under the microbial pesticide data requirements. This distinction is an advantage of VIF over transgenic approaches, since plant-incorporated protectants are subject to more stringent regulation than methods regulated by microbial pesticide data requirements. Notwithstanding, the stringency and intensity of regulation in the United States for field use are difficult to predict, suggesting that it would be wise to use disarmed viral vectors and common or innocuous species in a contained facility and ensure that seed produced is free from recombinant virus. The incorporation of a VIF strategy for citrus breeding in Spain is encouraging for broader regulatory approval (Velázquez et al., 2016). It will be particularly interesting to watch for the acceptance of the resulting varieties, since public perception and associated market obstacles to transgenic types of the technology may be as large a hurdle for biotechnology as government approval.
SUMMARY
Many breeding programs already rely upon genome-assisted approaches, such as genome-wide selection and MAS, to increase breeding efficiency. VIF is a promising new tool for accelerating research and breeding in crop species that show delayed flowering or are otherwise difficult to breed. It has the potential to circumvent a number of the problems of floral induction through horticultural and transgenic means. However, as outlined in the Outstanding Questions Box, interactions with regulatory agencies to clarify requirements and obstacles, and the subsequent development of regulation-friendly vectors and strains, as well as additional basic research on strain specificity and sexual transmission, are needed to fully understand its potential and limitations.
Acknowledgments
We thank Chris Wozniak of the Environmental Protection Agency and John Turner of the U.S. Department of Agriculture for providing information on their agencies’ regulations. We also thank past and present members of our laboratories for lively discussions.
Glossary
- MAS
marker-assisted selection
- VIGS
virus-induced gene silencing
- VIF
virus-induced flowering
- dCLCrV
disarmed cotton leaf crumple virus
- ALSV
Apple latent spherical virus
- CLBV
Citrus leaf blotch virus
- TRV
Tobacco rattle virus
Footnotes
This work was supported by Cotton Incorporated (cooperative agreement no. 10–768 to B.G.A.), the United States-Israel Binational Agricultural Research and Development Fund (project no. US–4535–12 to B.G.A.), the United Sorghum Checkoff Board (contract no. CI004–15 to B.G.A.), the Tree Biosafety and Genomics Research Cooperative at Oregon State University (to S.H.S.), the J. Frank Schmidt Family Charitable Foundation (to S.H.S.), Arborgen matching funds for the Consortium for Plant Biotechnology Research (to S.H.S.), the National Science Foundation Center for Advanced Forestry Systems (to S.H.S.), and the U.S. Department of Agriculture Biotechnology Risk Assessment Program (grant no. 2008–33120–19546 to S.H.S.).
Articles can be viewed without a subscription.
References
- Altpeter F, Springer NM, Bartley LE, Blechl AE, Brutnell TP, Citovsky V, Conrad LJ, Gelvin SB, Jackson DP, Kausch AP, et al. (2016) Advancing crop transformation in the era of genome editing. Plant Cell 28: 1510–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasino R. (2010) Seasonal and developmental timing of flowering. Plant J 61: 1001–1013 [DOI] [PubMed] [Google Scholar]
- Arnoult S, Brancourt-Hulmel M (2015) A review on Miscanthus biomass production and composition for bioenergy use: genotypic and environmental variability and implications for breeding. BioEnergy Res 8: 502–526 [Google Scholar]
- Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043 [DOI] [PubMed] [Google Scholar]
- Bolotin M. (1975) Photoperiodic induction of precocious flowering in a woody species Eucalyptus occidentalis Endl. Bot Gaz 136: 358–365 [Google Scholar]
- Bredeson JV, Lyons JB, Prochnik SE, Wu GA, Ha CM, Edsinger-Gonzales E, Grimwood J, Schmutz J, Rabbi IY, Egesi C, et al. (2016) Sequencing wild and cultivated cassava and related species reveals extensive interspecific hybridization and genetic diversity. Nat Biotechnol 34: 562–570 [DOI] [PubMed] [Google Scholar]
- Callahan A, Dardick C, Tosetti R, Lalli D, Scorza R (2015) 21st century approach to improving Burbank’s ‘stoneless’ plum. HortScience 50: 195–200 [Google Scholar]
- Carrillo-Tripp J, Shimada-Beltrán H, Rivera-Bustamante R (2006) Use of geminiviral vectors for functional genomics. Curr Opin Plant Biol 9: 209–215 [DOI] [PubMed] [Google Scholar]
- Ceballos H, Iglesias CA, Pérez JC, Dixon AG (2004) Cassava breeding: opportunities and challenges. Plant Mol Biol 56: 503–516 [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Sadava DE (2003) Plants, Genes, and Crop Biotechnology. Jones and Bartlett, Boston, MA [Google Scholar]
- Ding XS, Schneider WL, Chaluvadi SR, Mian MAR, Nelson RS (2006) Characterization of a Brome mosaic virus strain and its use as a vector for gene silencing in monocotyledonous hosts. Mol Plant Microbe Interact 19: 1229–1239 [DOI] [PubMed] [Google Scholar]
- eCFR (2016a) Electronic Code of Federal Regulations, Title 7, Subtitle B, Chapter III, Part 340. Introduction of organisms and products altered or produced through genetic engineering which are plant pests or which there is reason to believe are plant pests. http://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=34add3255b8763cd40759a68d46f087e&mc=true&r=PART&n=pt7.5.340 (August 15, 2016)
- eCFR (2016b) Electronic Code of Federal Regulations, Title 40, Chapter I, Subchapter E, Part 172, Subpart C. Notification for certain genetically modified microbial pesticides. http://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=5d257f9d8f5ec31ebdccc4de67bd7e49&ty=HTML&h=L&mc=true&n=sp40.26.172.c&r=SUBPART (August 15, 2016)
- Eldridge K, Davidson J, Harwood C, Wyk G (1993) Eucalyptus Domestication and Breeding. Clarendon Press, Oxford [Google Scholar]
- Endo T, Shimada T, Fujii H, Kobayashi Y, Araki T, Omura M (2005) Ectopic expression of an FT homolog from citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.). Transgenic Res 14: 703–712 [DOI] [PubMed] [Google Scholar]
- Flachowsky H, Le Roux PM, Peil A, Patocchi A, Richter K, Hanke MV (2011) Application of a high-speed breeding technology to apple (Malus × domestica) based on transgenic early flowering plants and marker-assisted selection. New Phytol 192: 364–377 [DOI] [PubMed] [Google Scholar]
- Flachowsky H, Szankowski I, Waidmann S, Peil A, Tränkner C, Hanke MV (2012) The MdTFL1 gene of apple (Malus × domestica Borkh.) reduces vegetative growth and generation time. Tree Physiol 32: 1288–1301 [DOI] [PubMed] [Google Scholar]
- Fofana IBF, Sangaré A, Collier R, Taylor C, Fauquet CM (2004) A geminivirus-induced gene silencing system for gene function validation in cassava. Plant Mol Biol 56: 613–624 [DOI] [PubMed] [Google Scholar]
- Freiman A, Shlizerman L, Golobovitch S, Yablovitz Z, Korchinsky R, Cohen Y, Samach A, Chevreau E, Le Roux PM, Patocchi A, et al. (2012) Development of a transgenic early flowering pear (Pyrus communis L.) genotype by RNAi silencing of PcTFL1-1 and PcTFL1-2. Planta 235: 1239–1251 [DOI] [PubMed] [Google Scholar]
- Griffin AR, Whiteman P, Rudge T, Burgess IP, Moncur M (1993) Effect of paclobutrazol on flower-bud production and vegetative growth in 2 species of Eucalyptus. Can J For Res 23: 640–647 [Google Scholar]
- Hanano S, Goto K (2011) Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell 23: 3172–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardner CM, Evans K, Brien C, Bliss F, Peace C (2016) Genetic architecture of apple fruit quality traits following storage and implications for genetic improvement. Tree Genet Genomes 12: 1–21 [Google Scholar]
- Hileman LC, Drea S, Martino G, Litt A, Irish VF (2005) Virus-induced gene silencing is an effective tool for assaying gene function in the basal eudicot species Papaver somniferum (opium poppy). Plant J 44: 334–341 [DOI] [PubMed] [Google Scholar]
- Ho WWH, Weigel D (2014) Structural features determining flower-promoting activity of Arabidopsis FLOWERING LOCUS T. Plant Cell 26: 552–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenicka H, Lehnhardt D, Briones V, Nilsson O, Fladung M (2016) Low temperatures are required to induce the development of fertile flowers in transgenic male and female early flowering poplar (Populus tremula L.). Tree Physiol 36: 667–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenicka H, Lehnhardt D, Nilsson O, Hanelt D, Fladung M (2014) Successful crossings with early flowering transgenic poplar: interspecific crossings, but not transgenesis, promoted aberrant phenotypes in offspring. Plant Biotechnol J 12: 1066–1074 [DOI] [PubMed] [Google Scholar]
- Hsu CY, Liu Y, Luthe DS, Yuceer C (2006) Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. Plant Cell 18: 1846–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal MJ, Reddy OUK, El-Zik KM, Pepper AE (2001) A genetic bottleneck in the ‘evolution under domestication’ of upland cotton Gossypium hirsutum L. examined using DNA fingerprinting. Theor Appl Genet 103: 547–554 [Google Scholar]
- Iwata H, Minamikawa MF, Kajiya-Kanegae H, Ishimori M, Hayashi T (2016) Genomics-assisted breeding in fruit trees. Breed Sci 66: 100–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen E, Edwards MC, Hampton RO (1994) Seed transmission of viruses: current perspectives. Annu Rev Phytopathol 32: 363–386 [Google Scholar]
- Kaeppler SM, Kaeppler HF, Rhee Y (2000) Epigenetic aspects of somaclonal variation in plants. Plant Mol Biol 43: 179–188 [DOI] [PubMed] [Google Scholar]
- Kimber CT, Dahlberg JA, Kresovich S (2013) The gene pool of Sorghum bicolor and its improvement. In Paterson HA, ed, Genomics of the Saccharinae. Springer, New York, pp 23–41 [Google Scholar]
- Klein RR, Miller FR, Klein PE, Burke JJ (2013) Registration of partially converted germplasm from 44 accessions of the USDA-ARS Ethiopian and Sudanese sorghum collections. J Plant Regist 7: 368–372 [Google Scholar]
- Klocko AL, Ma C, Robertson S, Esfandiari E, Nilsson O, Strauss SH (2016) FT overexpression induces precocious flowering and normal reproductive development in Eucalyptus. Plant Biotechnol J 14: 808–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoda N, Hayashi H, Suzuki M, Igarashi M, Hatsuyama Y, Kidou S, Igasaki T, Nishiguchi M, Yano K, Shimizu T, et al. (2010) Molecular characterization of FLOWERING LOCUS T-like genes of apple (Malus × domestica Borkh.). Plant Cell Physiol 51: 561–575 [DOI] [PubMed] [Google Scholar]
- Lifschitz E, Ayre BG, Eshed Y (2014) Florigen and anti-florigen: a systemic mechanism for coordinating growth and termination in flowering plants. Front Plant Sci 5: 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz E, Eviatar T, Rozman A, Shalit A, Goldshmidt A, Amsellem Z, Alvarez JP, Eshed Y (2006) The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci USA 103: 6398–6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MK, Belanger H, Lee YJ, Varkonyi-Gasic E, Taoka K, Miura E, Xoconostle-Cázares B, Gendler K, Jorgensen RA, Phinney B, et al. (2007) FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. Plant Cell 19: 1488–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Xie K, Jia Q, Zhao J, Chen T, Li H, Wei X, Diao X, Hong Y, Liu Y (2016) Foxtail mosaic virus-induced gene silencing in monocot plants. Plant Physiol 171: 1801–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Lusser M, Davies HV (2013) Comparative regulatory approaches for groups of new plant breeding techniques. N Biotechnol 30: 437–446 [DOI] [PubMed] [Google Scholar]
- McGarry RC, Ayre BG (2012a) Manipulating plant architecture with members of the CETS gene family. Plant Sci 188-189: 71–81 [DOI] [PubMed] [Google Scholar]
- McGarry RC, Ayre BG (2012b) Geminivirus-mediated delivery of florigen promotes determinate growth in aerial organs and uncouples flowering from photoperiod in cotton. PLoS ONE 7: e36746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry RC, Prewitt S, Ayre BG (2013) Overexpression of FT in cotton affects architecture but not floral organogenesis. Plant Signal Behav 8: e23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry RC, Prewitt SF, Culpepper S, Eshed Y, Lifschitz E, Ayre BG (2016) Monopodial and sympodial branching architecture in cotton is differentially regulated by the Gossypium hirsutum SINGLE FLOWER TRUSS and SELF-PRUNING orthologs. New Phytol 212: 244–258 [DOI] [PubMed] [Google Scholar]
- Mei Y, Zhang C, Kernodle BM, Hill JH, Whitham SA (2016) A Foxtail mosaic virus vector for virus-induced gene silencing in maize. Plant Physiol 171: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaggia AA, Piacezzi AL, Grattapaglia D (2005) Genetic mapping of Eef1, a major effect QTL for early flowering in Eucalyptus grandis. Tree Genet Genomes 1: 79–84 [Google Scholar]
- Morris GP, Ramu P, Deshpande SP, Hash CT, Shah T, Upadhyaya HD, Riera-Lizarazu O, Brown PJ, Acharya CB, Mitchell SE, et al. (2013) Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proc Natl Acad Sci USA 110: 453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RL, Klein RR, Morishige DT, Brady JA, Rooney WL, Miller FR, Dugas DV, Klein PE, Mullet JE (2011) Coincident light and clock regulation of pseudoresponse regulator protein 37 (PRR37) controls photoperiodic flowering in sorghum. Proc Natl Acad Sci USA 108: 16469–16474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH (2016) The National Institutes of Health Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules. http://osp.od.nih.gov/sites/default/files/resources/NIH_Guidelines.pdf
- Paterson AH, Boman RK, Brown SM, Chee PW, Gannaway JR, Gingle AR, May OL, Smith CW (2004) Reducing the genetic vulnerability of cotton. Crop Sci 44: 1900–1901 [Google Scholar]
- Paterson AH, Moore PH, Tew TL (2013) The gene pool of Saccharum species and their improvement. In Paterson HA, ed, Genomics of the Saccharinae. Springer, New York, pp 43–71 [Google Scholar]
- Racedo J, Gutiérrez L, Perera MF, Ostengo S, Pardo EM, Cuenya MI, Welin B, Castagnaro AP (2016) Genome-wide association mapping of quantitative traits in a breeding population of sugarcane. BMC Plant Biol 16: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. (2004) VIGS vectors for gene silencing: many targets, many tools. Annu Rev Plant Biol 55: 495–519 [DOI] [PubMed] [Google Scholar]
- Robinson AF. (2007) Reniform in U.S. cotton: when, where, why, and some remedies. Annu Rev Phytopathol 45: 263–288 [DOI] [PubMed] [Google Scholar]
- Sacks EJ, Juvik JA, Lin Q, Stewart JR, Yamada T (2013) The gene pool of Miscanthus species and its improvement. In Paterson HA, ed, Genomics of the Saccharinae. Springer, New York, pp 73–101 [Google Scholar]
- Saha S, Jenkins JN, Wu J, McCarty JC, Stelly DM (2008) Genetic analysis of agronomic and fibre traits using four interspecific chromosome substitution lines in cotton. Plant Breed 127: 612–618 [Google Scholar]
- Sayre R, Beeching JR, Cahoon EB, Egesi C, Fauquet C, Fellman J, Fregene M, Gruissem W, Mallowa S, Manary M, et al. (2011) The BioCassava plus program: biofortification of cassava for sub-Saharan Africa. Annu Rev Plant Biol 62: 251–272 [DOI] [PubMed] [Google Scholar]
- Scofield SR, Nelson RS (2009) Resources for virus-induced gene silencing in the grasses. Plant Physiol 149: 152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sein CC, Mitlohner R (2011) Eucalyptus urophylla. In Blake ST, ed, Ecology and Silviculture. CIFOR, Bogor, Indonesia, pp 1–16 [Google Scholar]
- Senthil-Kumar M, Mysore KS (2011) New dimensions for VIGS in plant functional genomics. Trends Plant Sci 16: 656–665 [DOI] [PubMed] [Google Scholar]
- Shalit A, Rozman A, Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y, Lifschitz E (2009) The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc Natl Acad Sci USA 106: 8392–8397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan C, Dardick C, Callahan A, Scorza R (2012) Plum (Prunus domestica) trees transformed with poplar FT1 result in altered architecture, dormancy requirement, and continuous flowering. PLoS ONE 7: e40715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss SH, Sax JK (2016) Ending event-based regulation of GMO crops. Nat Biotechnol 34: 474–477 [DOI] [PubMed] [Google Scholar]
- Tränkner C, Lehmann S, Hoenicka H, Hanke MV, Fladung M, Lenhardt D, Dunemann F, Gau A, Schlangen K, Malnoy M, et al. (2010) Over-expression of an FT-homologous gene of apple induces early flowering in annual and perennial plants. Planta 232: 1309–1324 [DOI] [PubMed] [Google Scholar]
- USDA (2011) APHIS confirmation of the regulatory status of null segregant (NS) lines derived from genetically engineered (GE) plants in an accelerated tobacco breeding program. https://www.aphis.usda.gov/biotechnology/downloads/reg_loi/Dr%20Ramsey%20S%20Lewis%20NCSC%20Final.pdf (August 15, 2016)
- USDA (2014) Request for confirmation that [ ] potato is not a regulated article. https://www.aphis.usda.gov/biotechnology/downloads/reg_loi/aphis_response_cellectis_potato.pdf (August 15, 2016)
- van Nocker S, Gardiner SE (2014) Breeding better cultivars, faster: applications of new technologies for the rapid deployment of superior horticultural tree crops. Hortic Res 1: 14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez K, Agüero J, Vives MC, Aleza P, Pina JA, Moreno P, Navarro L, Guerri J (2016) Precocious flowering of juvenile citrus induced by a viral vector based on Citrus leaf blotch virus: a new tool for genetics and breeding. Plant Biotechnol J 14: 1976–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TP, Stelly DM, Smith W, Robinson F, Stewart JM, Weaver DB, Ulloa M, Thaxton P, Percy R, Chee P, et al. (2009) Status of the USA cotton germplasm collection and crop vulnerability. Genet Resour Crop Evol 56: 507–532 [Google Scholar]
- Weigl K, Wenzel S, Flachowsky H, Peil A, Hanke MV (2015) Integration of BpMADS4 on various linkage groups improves the utilization of the rapid cycle breeding system in apple. Plant Biotechnol J 13: 246–258 [DOI] [PubMed] [Google Scholar]
- Wenzel S, Flachowsky H, Hanke MV (2013) The fast-track breeding approach can be improved by heat-induced expression of the FLOWERING LOCUS T genes from poplar (Populus trichocarpa) in apple (Malus × domestica Borkh.). Plant Cell Tissue Organ Cult 115: 127–137 [Google Scholar]
- Williams DR, Potts BM, Smethurst PJ (2003) Promotion of flowering in Eucalyptus nitens by paclobutrazol was enhanced by nitrogen fertilizer. Can J For Res 33: 74–81 [Google Scholar]
- Wolabu TW, Zhang F, Niu L, Kalve S, Bhatnagar-Mathur P, Muszynski MG, Tadege M (2016) Three FLOWERING LOCUS T-like genes function as potential florigens and mediate photoperiod response in sorghum. New Phytol 210: 946–959 [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Kishigami R, Yoshikawa N (2014) Reduced generation time of apple seedlings to within a year by means of a plant virus vector: a new plant-breeding technique with no transmission of genetic modification to the next generation. Plant Biotechnol J 12: 60–68 [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Sasaki S, Yamagata K, Komori S, Nagase M, Wada M, Yamamoto T, Yoshikawa N (2011) Promotion of flowering and reduction of a generation time in apple seedlings by ectopical expression of the Arabidopsis thaliana FT gene using the Apple latent spherical virus vector. Plant Mol Biol 75: 193–204 [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Yoshikawa N (2011) Expression of FLOWERING LOCUS T from Arabidopsis thaliana induces precocious flowering in soybean irrespective of maturity group and stem growth habit. Planta 233: 561–568 [DOI] [PubMed] [Google Scholar]
- Yuan C, Li C, Yan L, Jackson AO, Liu Z, Han C, Yu J, Li D (2011) A high throughput barley stripe mosaic virus vector for virus induced gene silencing in monocots and dicots. PLoS ONE 6: e26468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD. (2008) Leaf-produced floral signals. Curr Opin Plant Biol 11: 541–547 [DOI] [PubMed] [Google Scholar]
- Zhang H, Harry DE, Ma C, Yuceer C, Hsu CY, Vikram V, Shevchenko O, Etherington E, Strauss SH (2010) Precocious flowering in trees: the FLOWERING LOCUS T gene as a research and breeding tool in Populus. J Exp Bot 61: 2549–2560 [DOI] [PubMed] [Google Scholar]