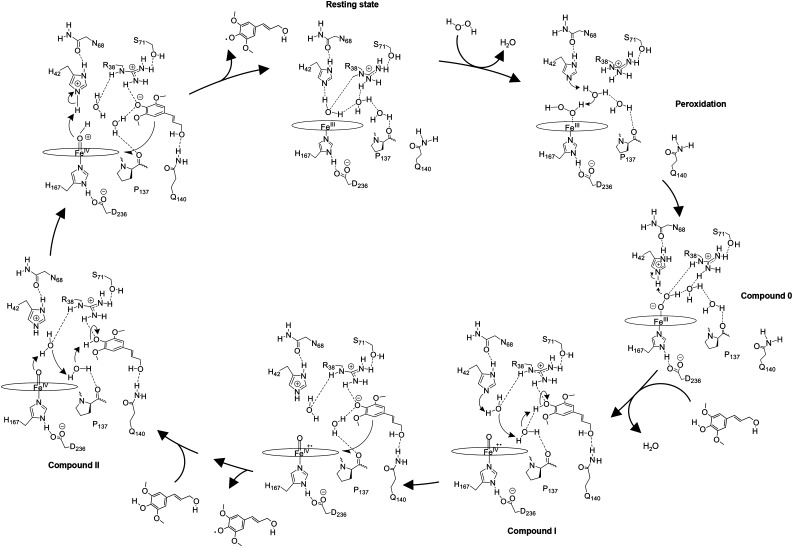
Figure 11.
Proposed catalytic reaction mechanism of PviPRX9. In the first step, H2O2 displaces a water molecule from the active site in its resting state and reacts with His-42 to generate a hydroperoxide molecule via a water-mediated proton transfer. Next, the hydroperoxide molecule coordinates Fe(III) heme to generate Fe(III)-hydroperoxo heme (compound 0). In the second step, the hydroperoxo ligand is protonated by His-42 to generate first water and an Fe(IV)-oxo heme π-cation radical (compound I). Then, a monolignol binds to PviPRX9, displacing a water. His-42 deprotonates the monolignyl 4-hydroxy through a water-mediated proton-shuttling mechanism, generating the negatively charged monolignol that donates an electron to compound I to generate the first monolignol radical and Fe(IV)-oxo heme (compound II). In the third step, a second monolignol binds and is deprotonated through a water-mediated proton shuttle. Next, an electron is transferred to the heme from the deprotonated monolignol and His-42 protonates the Fe(IV)-hydroxo heme, generating the second water molecule along with the second monolignol radical, which diffuses from the pocket, returning PviPRX9 to its resting state.