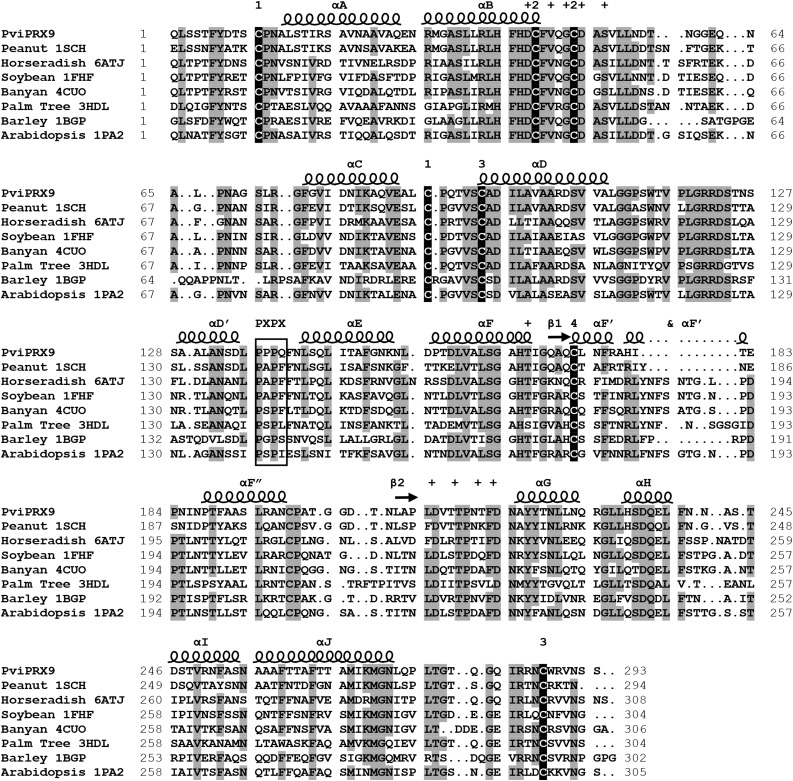
Figure 3.
Structural alignment of PviPRX9 with seven CIIIPRXs from the Research Collaboratory for Structural Bioinformatics PDB. Secondary structural elements were labeled with helices indicated by spirals and β-sheets indicated by arrows. The secondary structure was letter mapped according to the system laid out by Schuller et al. (1996). Residues coordinating with the two conserved Ca2+ atoms are indicated with plus signs. The conserved P-X-P-X motif is outlined with a box. The Cys residues participating in conserved disulfides are numbered according to the corresponding Cys. Amino acids with 75% or greater identity in the alignment are highlighted in gray, and the conserved Cys residues are highlighted in black.