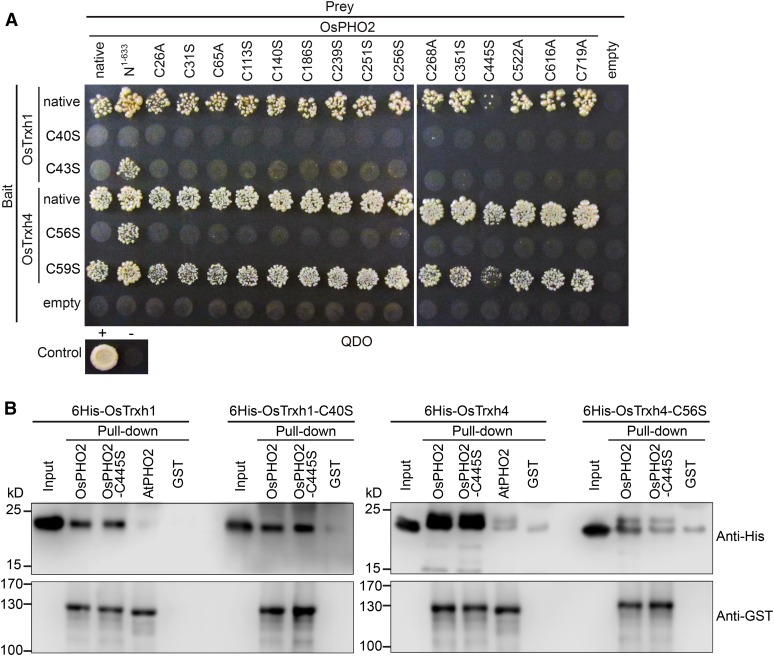
Figure 2.
The Cys-445 of OsPHO2 and the N terminus Cys in “WCGPC” motif of Trxhs are important for the interaction of OsPHO2 with OsTrxh1 and OsTrxh4. A, Y2H analyses of the Cys site-mutated OsPHO2 with OsTrxh1/h4. For prey, the Cys residues were changed to Ala or Ser and indicated as C26A, C31S, C65A, C113S, C140S, C186S, C251S, C256S, C268A, C351S, C445S, C522A, C616A, and C719A, respectively. For bait, the Cys residues in the “WCGPC” motif of OsTrxh1/h4 were changed to Ser and indicated as C40S, C43S, C56S, and C59S, respectively. The OsPHO2, OsTrxh1, and OsTrxh4 without mutation were indicated as “native,” respectively. B, In vitro interaction of 6His-tagged OsTrxh1, OsTrxh1-C40S, OsTrxh4, and OsTrxh4-C56S with GST-tagged OsPHO2, OsPHO2-C445S, and AtPHO2. GST alone was used as a control for nonspecific binding. Anti-His and anti-GST antibodies were used to detect OsTrxh1/h4 and PHO2 proteins, respectively.