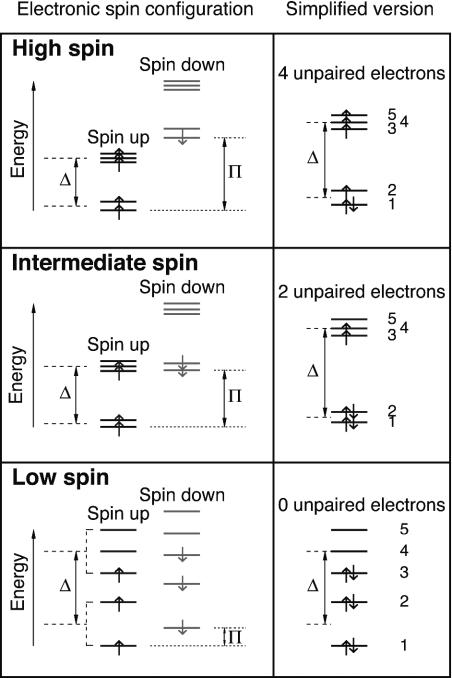
Fig. 4.
Schematic diagrams of electronic spin configuration of Fe2+ in the A site of perovskite based on crystal field theory (4). On the right are the simplified pictures that are commonly found in the literature. The 3d subshell of iron contains five orbitals, each capable of accommodating two electrons with opposite spins. The energy difference between the opposite spins on each orbital (dotted lines) is called spin-pairing energy (π). According to the crystal field theory, in a cubic environment, the 3d orbitals split into the t2g and eg levels (dashed lines). The energy difference between the two levels is called crystal field splitting energy (Δ). In a noncubic environment, additional splitting occurs between the t2g levels and between the eg levels, resulting in five different energy levels. At ambient condition, the 1st spin-down level is higher than the 5th spin-up level. Only one electron is forced to pair. Fe2+ has the largest number of unpaired electrons (high-spin state). Upon compression, crystal field splitting energy increases with increasing density (decreasing ligand bond lengths) and possibly increasing degree of distortion. Given fixed spin-pairing energy (5), certain lower spin-down levels will cross higher spin-up levels. When the 2nd spin-down level crosses the 5th spin-up level, the electron at the 5th orbital will switch spin and move to the 2nd orbital (intermediate-spin state, intermediate number of unpaired electrons). When the 3rd spin-down level crosses the 4th spin-up level, the electron at the 4th orbital will switch spin and move to the 3rd orbital (low-spin state, smallest number of unpaired electrons). In other words, each iron species goes through two pressure-induced partial spin-pairing transitions: Upon the first partial spin-pairing transition the number of unpaired 3d electrons drops to two, forming the intermediate-spin state. After the second partial transition, the number becomes zero, reaching the final low-spin state. Similar diagrams can be drawn for Fe3+ in either the A or the B site.