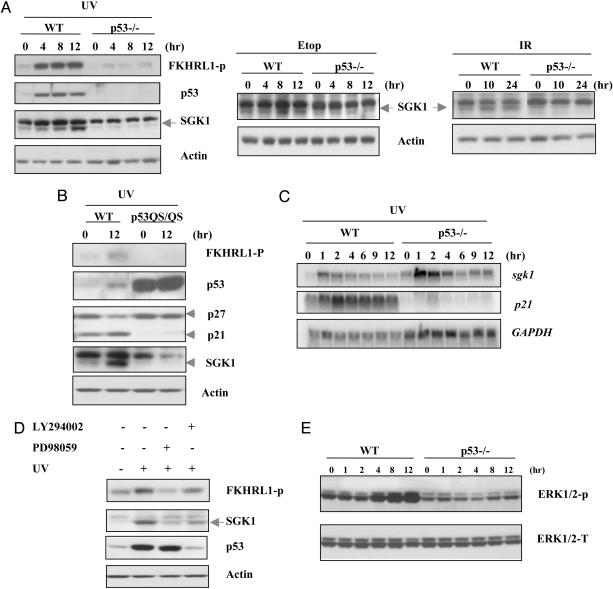
Fig. 3.
p53-dependent induction of SGK1 in response to DNA damage through the ERK1/2 pathway. (A) WT and p53–/–MEFs were treated with UV radiation (30 J/m2), etoposide (Etop, 0.5 μM), or γ radiation (10 Gy). Western blotting of cell lysates was carried out to detect phospho-FKHRL1 (which only showed in UV treatment), p53, and SGK1 at the indicated time points. Actin was used as the loading control. (B) p53 transactivation domain was required for SGK1 induction. Transformed WT and p53QS/QS MEFs were exposed to UV radiation (30 J/m2). Cell lysates were prepared 12 h after treatment, and Western blotting was performed by using antibodies against phospho-FKHRL1, p53, p27, p21, and SGK1. (C) p53-dependent SGK1 induction in response to UV radiation is through posttranslational regulation. WT and p53–/–MEFs were exposed to UV radiation (30 J/m2) for indicated times, and Northern blotting of total RNA was carried out to detect murine sgk1 and p21 transcripts. Full-length murine sgk1 was used as a hybridization probe. GAPDH was used as the loading control. (D) ERK1/2 dependence of posttranslational SGK1 induction by p53. WT MEFs were pretreated with the PI3K inhibitor LY294002 (10 μM) or the MEK1 inhibitor PD98059 (50 μM) for 1 h before treatment with UV radiation (30 J/m2) for another 8 h. Cell lysates were analyzed by Western blotting by using antibodies specifictothe indicated proteins. (E) Time course of ERK1/2 activation after UV damage (30 J/m2) in WT and p53–/–MEFs. Cell lysates were prepared at the indicated time points after UV exposure, and phosphorylated ERK (ERK1/2-P) was detected by using phospho-specific anti-ERK antibody. Total ERK (ERK1/2K-T) was assessed as the loading control.